At a certain temperature the rate of this reaction is first order in NH, with a rate constant of 1.83 s 2NH, (g) - N, (g) + 3H, (g) Suppose a vessel contains NH, at a concentration of 1.42M. Calculate the concentration of NH, in the vessel 0.710 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. | M Submit Assignmer Continue © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy CenterI Accessibilit 43,203 FEB 18 CH.9 PE ASSESS MacBook Air
At a certain temperature the rate of this reaction is first order in NH, with a rate constant of 1.83 s 2NH, (g) - N, (g) + 3H, (g) Suppose a vessel contains NH, at a concentration of 1.42M. Calculate the concentration of NH, in the vessel 0.710 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. | M Submit Assignmer Continue © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy CenterI Accessibilit 43,203 FEB 18 CH.9 PE ASSESS MacBook Air
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.50QE
Related questions
Question
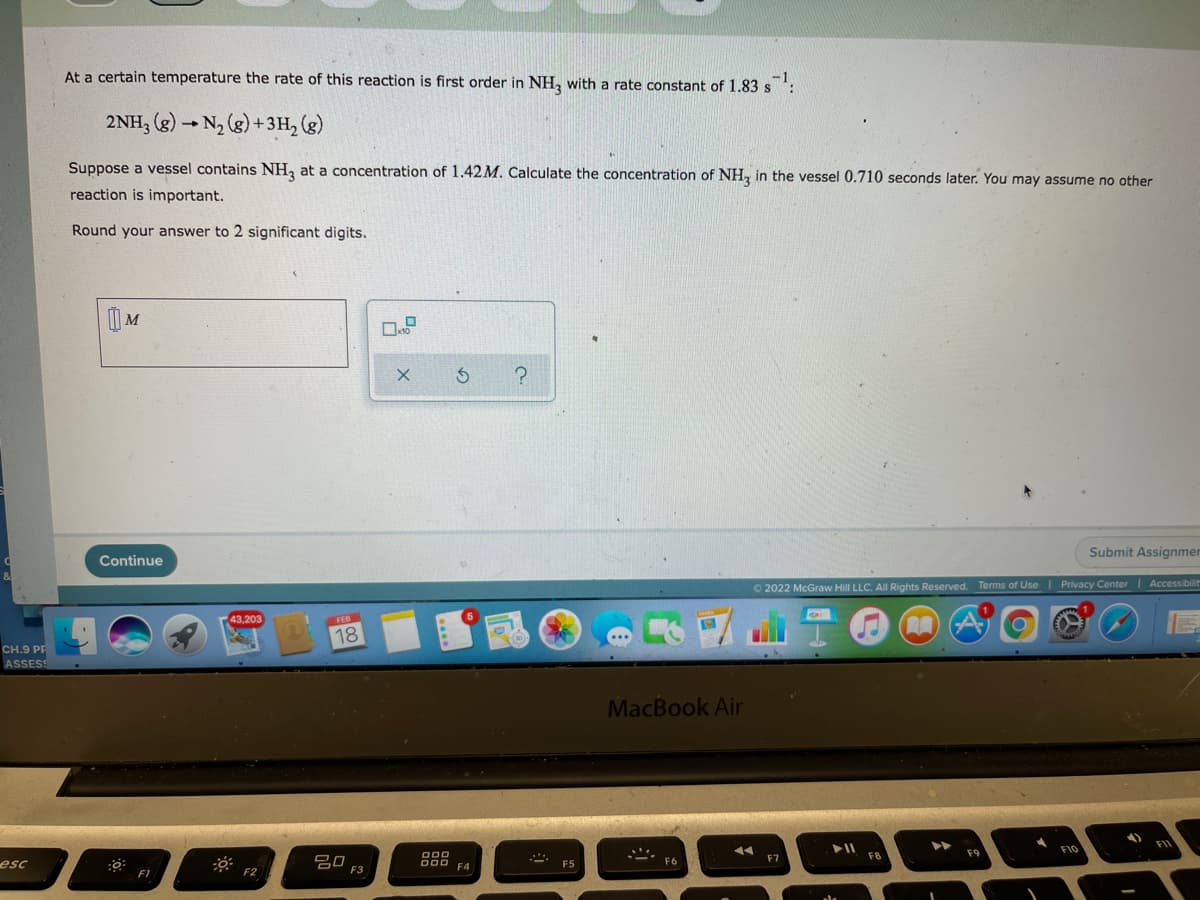
Transcribed Image Text:At a certain temperature the rate of this reaction is first order in NH, with a rate constant of 1.83 s
2NH, (g) - N2 (g) +3H, (g)
Suppose a vessel contains NH, at a concentration of 1.42M. Calculate the concentration of NH, in the vessel 0.710 seconds later. You may assume no other
reaction is important.
Round your answer to 2 significant digits.
| M
Submit Assignmer
Continue
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center| Accessibilit
43,203
FEB
18
CH.9 PF
ASSESS
MacBook Air
FIO
F9
D00
D00 F4
F7
F8
吕口
F3
esc
F5
F6
F1
F2
3)
Expert Solution
Step 1
Chemical kinetics can be defined as the branch of chemistry that deals with rates of chemical reactions.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning