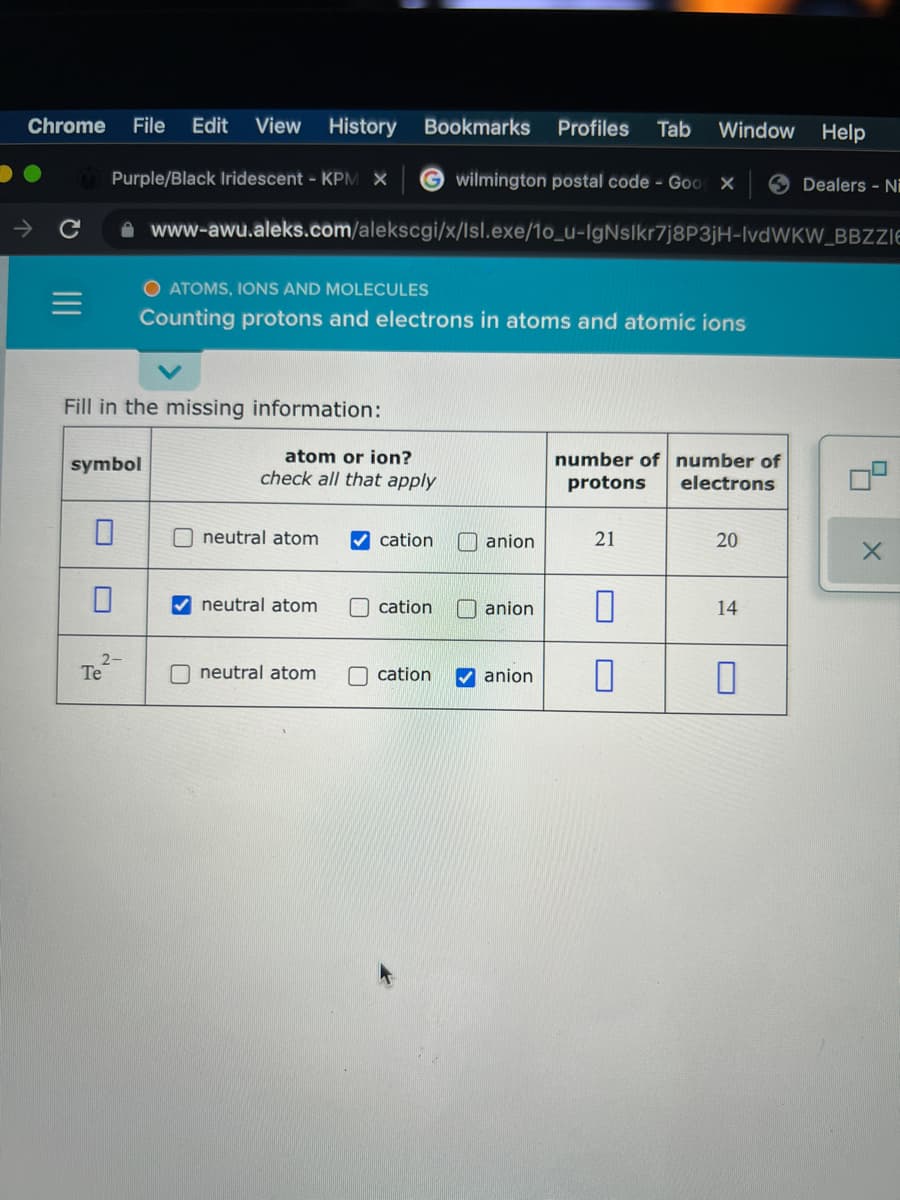
Fill in the missing information: atom or ion? check all that apply symbol number of number of protons electrons O neutral atom cation anion 21 20 neutral atom O cation anion 14 2- Te neutral atom cation anion
Fill in the missing information: atom or ion? check all that apply symbol number of number of protons electrons O neutral atom cation anion 21 20 neutral atom O cation anion 14 2- Te neutral atom cation anion
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 34PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View History Bookmarks
Profiles
Tab
Window
Help
Purple/Black Iridescent - KPM X
wilmington postal code - Goo X
Dealers - Ni
A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZIE
O ATOMS, IONS AND MOLECULES
Counting protons and electrons in atoms and atomic ions
Fill in the missing information:
symbol
atom or ion?
number of number of
check all that apply
protons
electrons
O neutral atom
V cation
anion
21
20
neutral atom
O cation
O anion
14
Te
O neutral atom
cation
V anion
Expert Solution
Step 1
Complete the given table :
a) cation ; number of protons = 21 ; number of electrons = 20 ; smybol = ?
b) neutral atom ; number of protons = ? ; number of electrons = 14 ; smybol = ?
c) symbol = Te2- ; anion ; number of protons = ? ; number of electrons = ?
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning