Atorvastatin (Lipitor) is a popular treatment for high cholesterol. See "The Importance of Hydrogen Bonding in Drug-Receptor Interactions" One synthesis of atorvastatin involves the following enolate reaction. Draw the predominant product of this reaction, which gives an overall yield of 90%. Но 1. 2 Equiv. LDA, MgBr, -78°C NH 3. H,O There are a couple of noteworthy aspects to this reaction. First, MgBr, is added to ex- change with Li and make Mg enolates. This is helpful for controlling stereochemistry. Notice that the starting material is chiral and that a single enantiomer is used. The prod- uct of this reaction is a 97:3 (94% ee) mixture of two enantiomers, not a racemic mix- ture. You don't have to be able to deduce which enantiomer is the predominant product, but be aware that being able to control the stereochemical outcome of a reaction by us- ing a single enantiomer of a chiral starting material can save time and resources in the large-scale synthesis of chiral drugs.
Atorvastatin (Lipitor) is a popular treatment for high cholesterol. See "The Importance of Hydrogen Bonding in Drug-Receptor Interactions" One synthesis of atorvastatin involves the following enolate reaction. Draw the predominant product of this reaction, which gives an overall yield of 90%. Но 1. 2 Equiv. LDA, MgBr, -78°C NH 3. H,O There are a couple of noteworthy aspects to this reaction. First, MgBr, is added to ex- change with Li and make Mg enolates. This is helpful for controlling stereochemistry. Notice that the starting material is chiral and that a single enantiomer is used. The prod- uct of this reaction is a 97:3 (94% ee) mixture of two enantiomers, not a racemic mix- ture. You don't have to be able to deduce which enantiomer is the predominant product, but be aware that being able to control the stereochemical outcome of a reaction by us- ing a single enantiomer of a chiral starting material can save time and resources in the large-scale synthesis of chiral drugs.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.80P
Related questions
Question
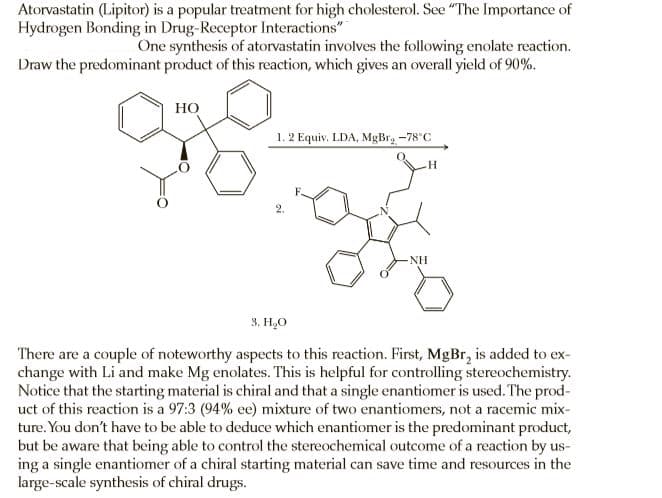
Transcribed Image Text:Atorvastatin (Lipitor) is a popular treatment for high cholesterol. See "The Importance of
Hydrogen Bonding in Drug-Receptor Interactions"
One synthesis of atorvastatin involves the following enolate reaction.
Draw the predominant product of this reaction, which gives an overall yield of 90%.
Но
1. 2 Equiv. LDA, MgBr, -78°C
NH
3. H,O
There are a couple of noteworthy aspects to this reaction. First, MgBr, is added to ex-
change with Li and make Mg enolates. This is helpful for controlling stereochemistry.
Notice that the starting material is chiral and that a single enantiomer is used. The prod-
uct of this reaction is a 97:3 (94% ee) mixture of two enantiomers, not a racemic mix-
ture. You don't have to be able to deduce which enantiomer is the predominant product,
but be aware that being able to control the stereochemical outcome of a reaction by us-
ing a single enantiomer of a chiral starting material can save time and resources in the
large-scale synthesis of chiral drugs.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
