Following are the final steps in one industrial synthesis of vitamin A acetate. H,SO, ÓH (1) (2) (3) Pseudoionone B-Ionone (racemic) OH Br Ph P, HBr (4) (5) (racemic) OCCH3 Vitamin A acetate (a) Propose a mechanism for the acid-catalyzed cyclization in Step 1. (b) Propose reagents to bring about Step 2. (c) Propose a mechanism for formation of the phosphonium salt in Step 4. (d) Propose reagents to bring about Step 3. (e) Show how Step 5 can be completed by a Wittig reaction.
Following are the final steps in one industrial synthesis of vitamin A acetate. H,SO, ÓH (1) (2) (3) Pseudoionone B-Ionone (racemic) OH Br Ph P, HBr (4) (5) (racemic) OCCH3 Vitamin A acetate (a) Propose a mechanism for the acid-catalyzed cyclization in Step 1. (b) Propose reagents to bring about Step 2. (c) Propose a mechanism for formation of the phosphonium salt in Step 4. (d) Propose reagents to bring about Step 3. (e) Show how Step 5 can be completed by a Wittig reaction.
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 35MP: One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to...
Related questions
Question
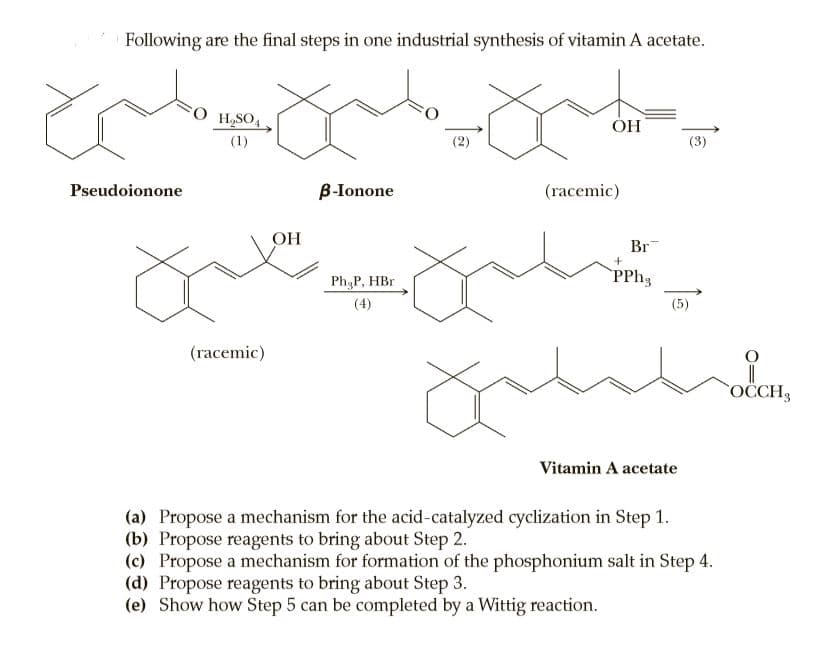
Transcribed Image Text:Following are the final steps in one industrial synthesis of vitamin A acetate.
H,SO,
ÓH
(1)
(2)
(3)
Pseudoionone
B-Ionone
(racemic)
OH
Br
Ph P, HBr
(4)
(5)
(racemic)
OCCH3
Vitamin A acetate
(a) Propose a mechanism for the acid-catalyzed cyclization in Step 1.
(b) Propose reagents to bring about Step 2.
(c) Propose a mechanism for formation of the phosphonium salt in Step 4.
(d) Propose reagents to bring about Step 3.
(e) Show how Step 5 can be completed by a Wittig reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 9 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning