Attached is the 13C-NMR Spectrum of 1,2,3,4-tetraphenylnapthalene (CDCl3) Can you provide (chemical shift (experimental), chemical shift (literature), assignment) Chemical shift δ(ppm) Experimental Chemical shift δ(ppm) Literature Assignmen
Attached is the 13C-NMR Spectrum of 1,2,3,4-tetraphenylnapthalene (CDCl3) Can you provide (chemical shift (experimental), chemical shift (literature), assignment) Chemical shift δ(ppm) Experimental Chemical shift δ(ppm) Literature Assignmen
Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.SE: Something Extra
Problem 37AP: The 1H NMR spectrum of bullvalene at 100 C consists only of a single peak at 4.22 . Explain.
Related questions
Question
Attached is the 13C-NMR Spectrum of 1,2,3,4-tetraphenylnapthalene (CDCl3)
Can you provide (chemical shift (experimental),
chemical shift (literature), assignment)
|
Chemical shift δ(ppm) Experimental |
Chemical shift δ(ppm) Literature |
Assignment |
|
|
||
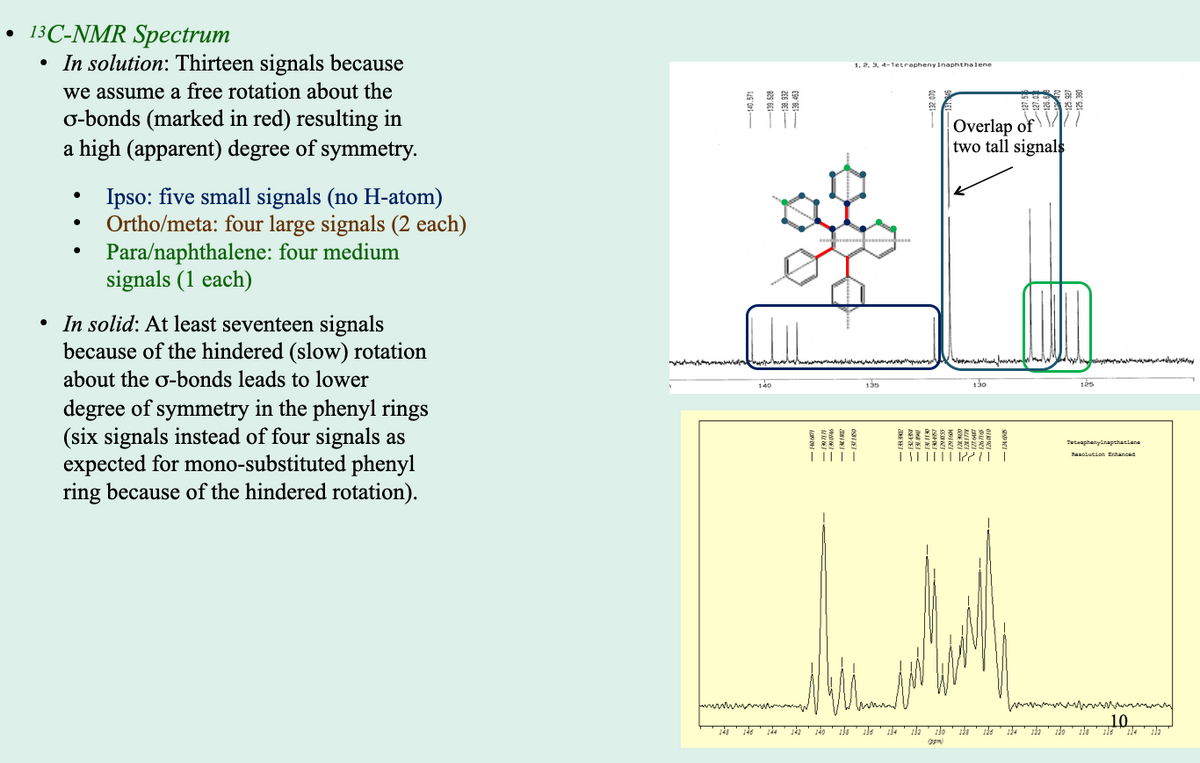
Transcribed Image Text:13C-NMR Spectrum
• In solution: Thirteen signals because
1. 2. 3, 4-Tetraphenyinaphthalene
we assume a free rotation about the
o-bonds (marked in red) resulting in
a high (apparent) degree of symmetry.
Overlap of
two tall signals
Ipso: five small signals (no H-atom)
Ortho/meta: four large signals (2 each)
Para/naphthalene: four medium
signals (1 each)
• In solid: At least seventeen signals
because of the hindered (slow) rotation
about the o-bonds leads to lower
140
135
130
degree of symmetry in the phenyl rings
(six signals instead of four signals as
expected for mono-substituted phenyl
ring because of the hindered rotation).
Teteaphanylnapshaniene
T|||||| |21
Sasotution Enhanced
wwwwmww
10
ils
140
132
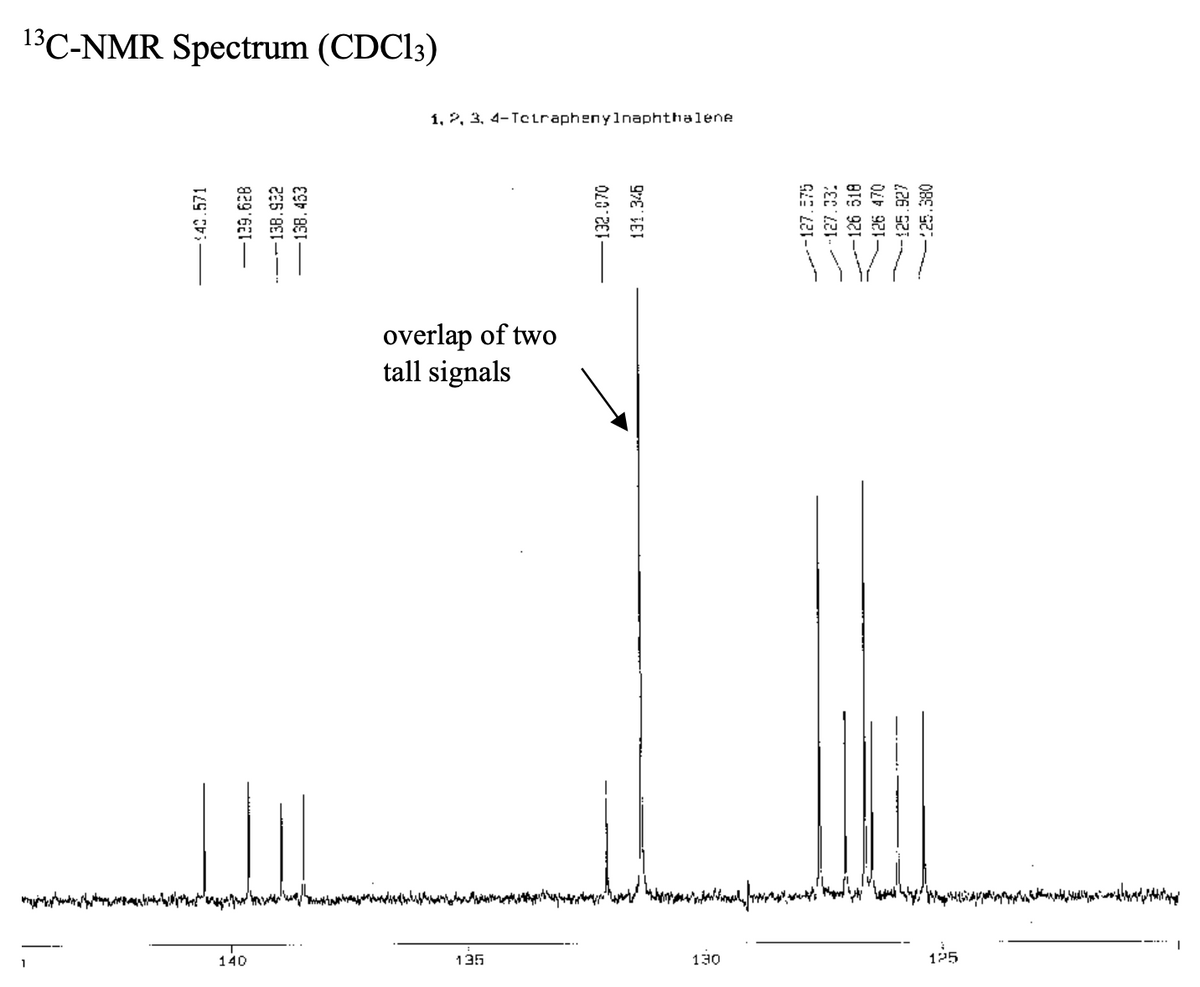
Transcribed Image Text:13C-NMR Spectrum (CDC13)
1, 2, 3, 4-Tciraphenylnaphthalene
overlap of two
tall signals
125
1
140
135
130
40.571
-129.628
-138.922
-138.453
F132.070
131.346
----127.E75
/127.33:
~-126 518
-126 470
125.927
:25.380
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

