Average net charge of +2 predominates: а. b. The predominant species is +H3N-CH-((CH2)3-NH-C-NH2-NH2+)-COO- : Average net charge of arginine is 0: C.
Average net charge of +2 predominates: а. b. The predominant species is +H3N-CH-((CH2)3-NH-C-NH2-NH2+)-COO- : Average net charge of arginine is 0: C.
Chapter10: Reconstitution Of Powdered Drugs
Section: Chapter Questions
Problem 7.5P
Related questions
Question
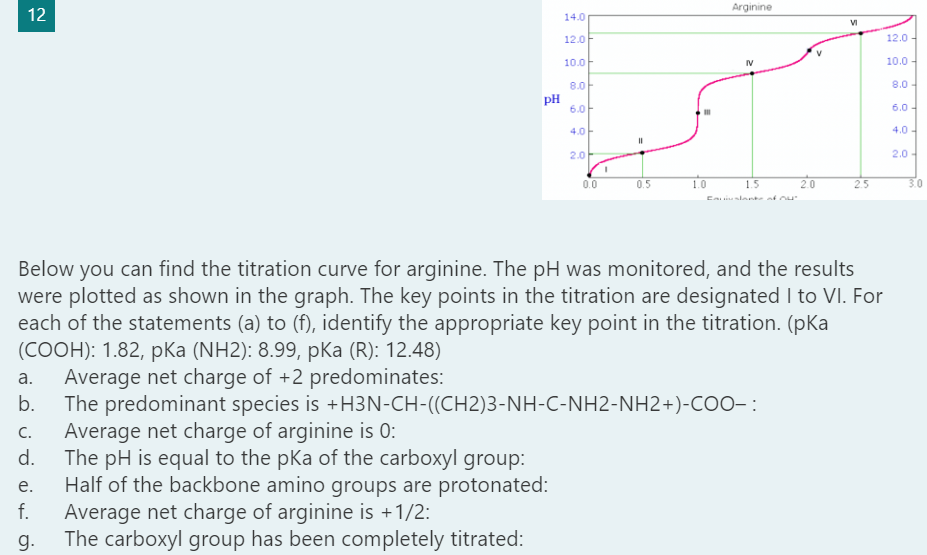
Transcribed Image Text:Arginine
12
14.0
VI
12.0F
12.0
10.0F
IV
10.0
8.0
8.0
pH
6.0
6.0
4.0-
4.0
2.0
2.0
0.0
0.5
1.0
1.5
2.0
2.5
3.0
Eguiyslonts of Ou
Below you can find the titration curve for arginine. The pH was monitored, and the results
were plotted as shown in the graph. The key points in the titration are designated I to VI. For
each of the statements (a) to (f), identify the appropriate key point in the titration. (pKa
(СООН): 1.82, рКa (NH2): 8.99, pKa (R): 12.48)
Average net charge of +2 predominates:
The predominant species is +H3N-CH-((CH2)3-NH-C-NH2-NH2+)-COO- :
Average net charge of arginine is 0:
d.
а.
b.
C.
The pH is equal to the pka of the carboxyl group:
Half of the backbone amino groups are protonated:
Average net charge of arginine is +1/2:
The carboxyl group has been completely titrated:
е.
f.
g.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage