B: Calculate the Normality of solutions containing the following with discussing your results:- 1) 8 gm /L of Na2CO3 (when the CO32 reacts with two protons). 2) 9.5 gm. /L of K2Cr2O7 (the Cr is reduced to Cr*3). Atomic weight (H= 1, C=12, O-16, Cl= 35.5, Na=23, K=39, Pb3207.19, Cr-52, Ag= 107.8)
B: Calculate the Normality of solutions containing the following with discussing your results:- 1) 8 gm /L of Na2CO3 (when the CO32 reacts with two protons). 2) 9.5 gm. /L of K2Cr2O7 (the Cr is reduced to Cr*3). Atomic weight (H= 1, C=12, O-16, Cl= 35.5, Na=23, K=39, Pb3207.19, Cr-52, Ag= 107.8)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 70AP
Related questions
Question
I need the answer as soon as possible
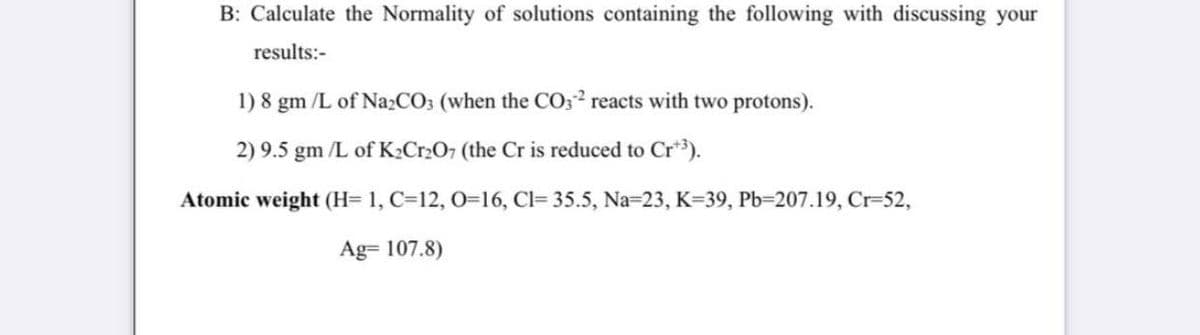
Transcribed Image Text:B: Calculate the Normality of solutions containing the following with discussing your
results:-
1)8 gm /L of Na2CO3 (when the CO32 reacts with two protons).
2) 9.5 gm /L of K2Cr2O7 (the Cr is reduced to Cr*3).
Atomic weight (H= 1, C=12, 0=16, Cl= 35.5, Na=23, K=39, Pb=207.19, Cr-52,
Ag= 107.8)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
