(b) What will happen if acetic acid (HC2H3O2) is added to water? O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by removal of water, C2H2O. O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by addition of water, H3C2H3O3. O Acetic acid is a strong acid and will ionize completely. O An equilibrium will form between its molecular form, HC2H3O2, and the ionized form, H* and C2H3O2 . O Acetic acid is a weak acid and will not ionize completely.
(b) What will happen if acetic acid (HC2H3O2) is added to water? O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by removal of water, C2H2O. O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by addition of water, H3C2H3O3. O Acetic acid is a strong acid and will ionize completely. O An equilibrium will form between its molecular form, HC2H3O2, and the ionized form, H* and C2H3O2 . O Acetic acid is a weak acid and will not ionize completely.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 7P
Related questions
Question
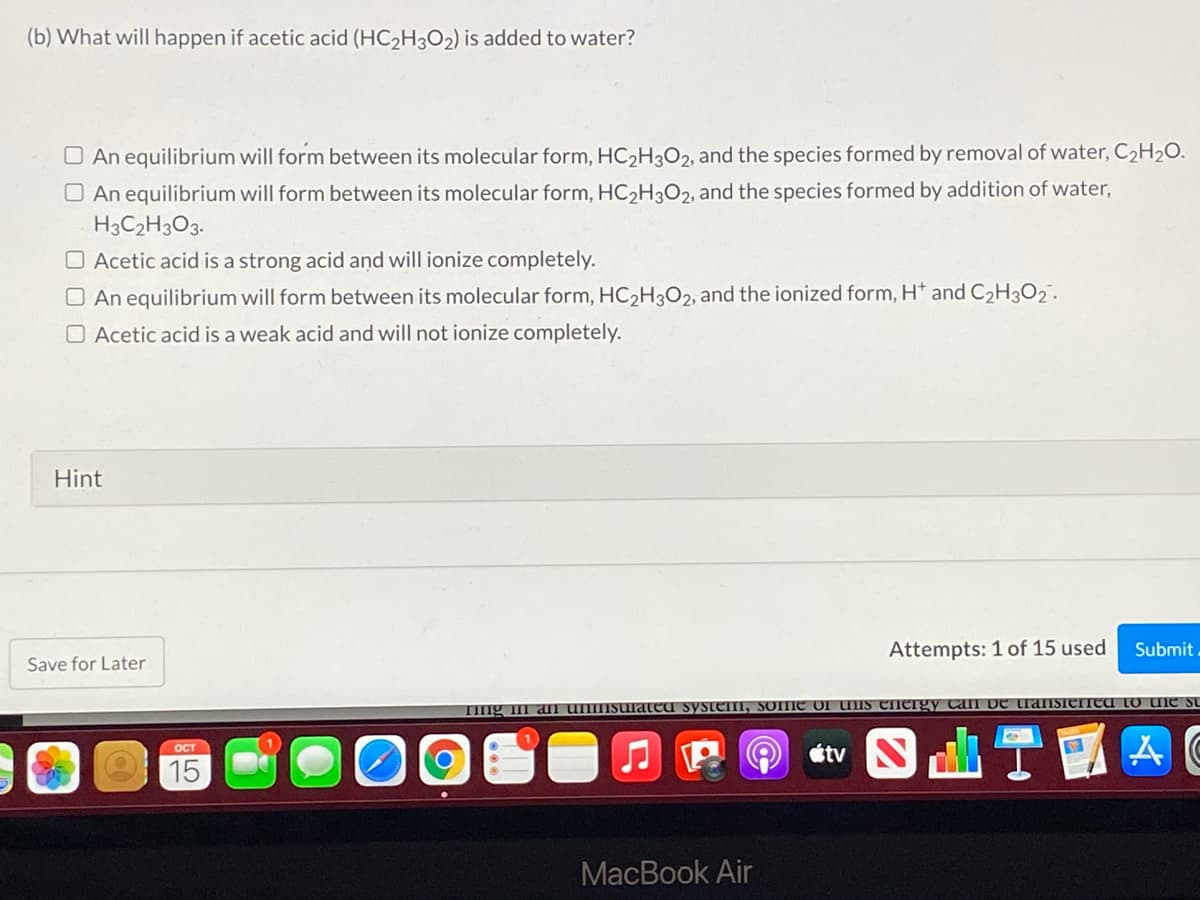
Transcribed Image Text:(b) What will happen if acetic acid (HC2H3O2) is added to water?
O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by removal of water, C2H2O.
O An equilibrium will form between its molecular form, HC2H3O2, and the species formed by addition of water,
H3C2H3O3.
O Acetic acid is a strong acid and will ionize completely.
O An equilibrium will form between its molecular form, HC2H3O2, and the ionized form, H* and C2H3O2.
O Acetic acid is a weak acid and will not ionize completely.
Hint
Attempts: 1 of 15 used
Submit
Save for Later
TIng I aI
system, SOme of UIS energy Can De transieIICU To une su
t ST國
ост
15
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning