B. In the following graphs, velocity represents the molecular speed, Velocity (m/s) (i) If the plot represents the velocity distribution of 1.0 L of O2 (g) at temperature of 2273 K versus 127 K, which plot corresponds to each temperature? Briefly explain If the plot represents the velocity distribution of 1.0 L of He (g) at STP and 1.0 L of Cl2 (g) (ii) at STP, which plot corresponds to which gas? Briefly explain. Relative number of molecules
B. In the following graphs, velocity represents the molecular speed, Velocity (m/s) (i) If the plot represents the velocity distribution of 1.0 L of O2 (g) at temperature of 2273 K versus 127 K, which plot corresponds to each temperature? Briefly explain If the plot represents the velocity distribution of 1.0 L of He (g) at STP and 1.0 L of Cl2 (g) (ii) at STP, which plot corresponds to which gas? Briefly explain. Relative number of molecules
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 7RQ
Related questions
Question
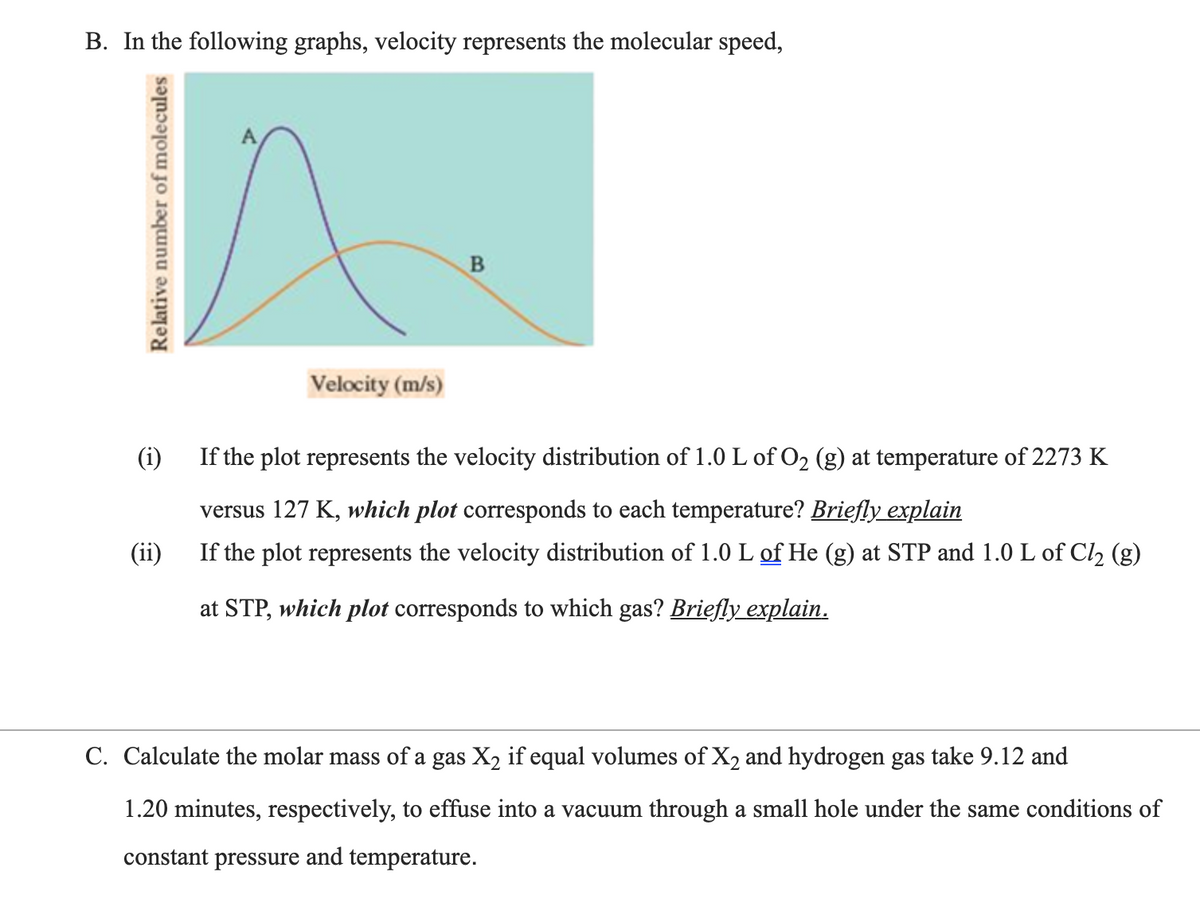
Transcribed Image Text:B. In the following graphs, velocity represents the molecular speed,
Velocity (m/s)
(i)
If the plot represents the velocity distribution of 1.0 L of O2 (g) at temperature of 2273 K
versus 127 K, which plot corresponds to each temperature? Briefly explain
(ii)
If the plot represents the velocity distribution of 1.0 L of He (g) at STP and 1.0 L of Cl, (g)
at STP, which plot corresponds to which gas? Briefly explain.
C. Calculate the molar mass of a gas X2 if equal volumes of X2 and hydrogen gas take 9.12 and
1.20 minutes, respectively, to effuse into a vacuum through a small hole under the same conditions of
constant pressure and temperature.
Relative number of molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning