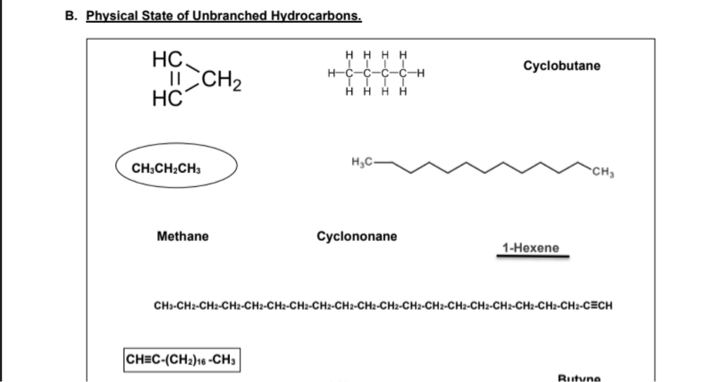
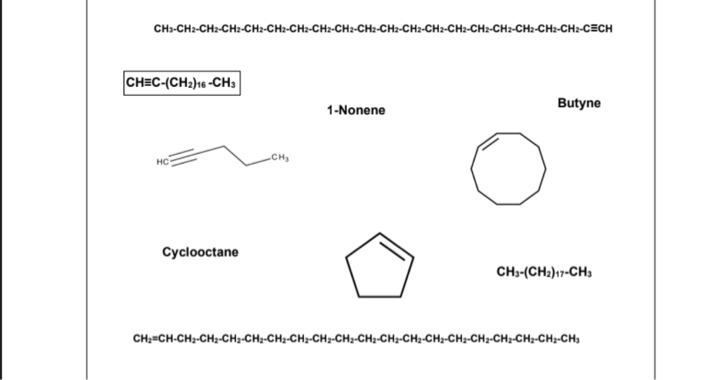
B. Physical State of Unbranched Hydrocarbons. HC. I CH2 HC° HH H H H-C-C-C-CH H H H H Сyclobutane H,C- CH,CH:CH, CH Methane Суclononane 1-Нехene CH-CHa-CH:-CH:-CH2-CH:-CH2-CH:-CH2-CH:-CH2-CH:-CH2-CH:-CHa-CH-CH:-CH-CH2-CECH
Physical State of Unbranched Hydrocarbons
Procedure:
- In the activity sheet, ENCIRCLE the compound if it is a gas, UNDERLINE the compound if it is a liquid, and BOX the compound if it is solid at room temperature.
- Unbranched
alkanes and unbranchedalkenes oralkynes with only one or triple bond containing 1 to 4 carbon atoms (C1-C4) are GASES at room temperature. - Unbranched alkanes and unbranched alkenes or alkynes with only one or triple bond containing 5 to 17 carbon atoms (C5-C17) are LIQUIDS at room temperature.
- Unbranched alkanes and unbranched alkenes or alkynes with only one or triple bond containing 18 or more carbon atoms (≥ C18) are SOLIDS at room temperature.
CH3CH2CH3 – It is a GAS because it is an unbranched alkane containing 3 carbon atoms.
1-Hexene – It is a LIQUID because it is an unbranched alkene containing 6 carbon atoms (if you will illustrate the structure) with only one double bond.
CH≡C-(CH2)16 -CH3 – It is a SOLID because it is an unbranched alkyne containing 19 carbon atoms with only one triple bond.
- Unbranched cycloalkanes and unbranched cycloalkenes with only one double bond containing 3 to 4 carbon atoms (C3-C4) are GASES at room temperature.
- Unbranched cycloalkanes and unbranched cycloalkenes with only one double bond containing 5 to 8 carbon atoms (C5-C8) are LIQUIDS at room temperature.
- Unbranched cycloalkanes and unbranched cycloalkenes with only one double bond containing 9 or more carbon atoms (≥ C9) are SOLIDS at room temperature.
Example:
Cyclopropane - It is a GAS because it is an unbranched cycloalkane containing 3 carbon atoms.
Cyclohexene - It is a LIQUID because it is an unbranched cycloalkene containing 6 carbon atoms with only one double bond.
Cyclodecane - It is a SOLID because it is an unbranched cycloalkane containing 10 carbon atoms.
Cyclononene It is a SOLID because it is an unbranched cycloalkene containing 9 carbon atoms with only one double bond.
(Note: We will not include Cycloalkynes because they are unstable and rarely found in nature)
Step by step
Solved in 3 steps with 21 images






