Melting or Boiling Point of Hydrocarbons Procedure: In the picture below, ENCIRCLE the compound that has higher boiling point or melting point for each of the following pairs. The longer the hydrocarbon chain or ring size the higher the melting or boiling points. 3. Cyclic structures have higher boiling or melting points than their non-cyclic counterparts with the same number of carbon atoms. These differences are due in large part to cyclic systems having more rigid and more symmetrical structures. Example: o Propane has higher melting or boiling point compared to ethane because propane has longer hydrocarbon chain which is composed of 3 carbon atoms in contrast to ethane which is composed only of 2 carbon atoms. o Cyclopentene has higher melting or boiling point compared to cyclobutene because cyclopentene has larger ring size which is composed of 5 carbon atoms in contrast to cyclobutene which is composed only of 4 carbon atoms. o Cyclopropane has higher melting or boiling point compared to propane because cyclic structures have higher boiling or melting points than their non-cyclic counterparts with the same number of carbon atoms. These differences are due in large part to cyclic systems having more rigid and more symmetrical structures.
Melting or Boiling Point of Hydrocarbons
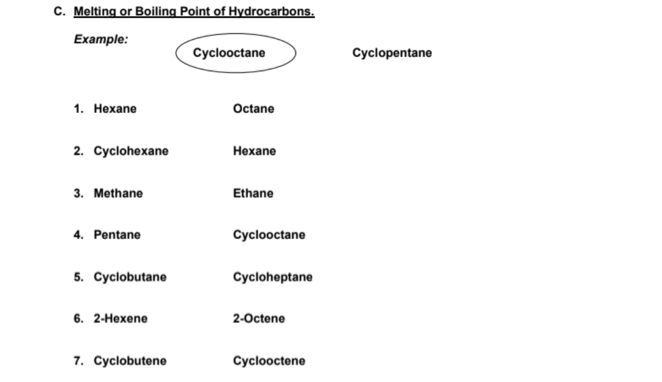
Procedure:
- In the picture below, ENCIRCLE the compound that has higher boiling point or melting point for each of the following pairs.
- The longer the hydrocarbon chain or ring size the higher the melting or boiling points. 3. Cyclic structures have higher boiling or melting points than their non-cyclic counterparts with the same number of carbon atoms. These differences are due in large part to cyclic systems having more rigid and more symmetrical structures.
Example:
o Propane has higher melting or boiling point compared to ethane because propane has longer hydrocarbon chain which is composed of 3 carbon atoms in contrast to ethane which is composed only of 2 carbon atoms.
o Cyclopentene has higher melting or boiling point compared to cyclobutene because cyclopentene has larger ring size which is composed of 5 carbon atoms in contrast to cyclobutene which is composed only of 4 carbon atoms.
o Cyclopropane has higher melting or boiling point compared to propane because cyclic structures have higher boiling or melting points than their non-cyclic counterparts with the same number of carbon atoms. These differences are due in large part to cyclic systems having more rigid and more symmetrical structures.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









