B. Propose a structure that will fit the given description. 1. skeletal structure of an alcohol C6H₁4O where the functional group is attached to a tertiary carbo 2. condensed structure of the constitutional isomer of C5H12 with the lowest boiling point 3. condensed structure of C4H10 that does not contain secondary carbons 4. Kekulé structures of the two functional isomers of C4H8O₂, one of which is a carboxylic acid without a tertiary carbon.
B. Propose a structure that will fit the given description. 1. skeletal structure of an alcohol C6H₁4O where the functional group is attached to a tertiary carbo 2. condensed structure of the constitutional isomer of C5H12 with the lowest boiling point 3. condensed structure of C4H10 that does not contain secondary carbons 4. Kekulé structures of the two functional isomers of C4H8O₂, one of which is a carboxylic acid without a tertiary carbon.
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 13EQ: This is done by removing an unshared pair from each of two adjacent atoms and adding one electron...
Related questions
Question
Please answer 1 to 3 if possible. Or if not, at least 1 and 2. Thanks!
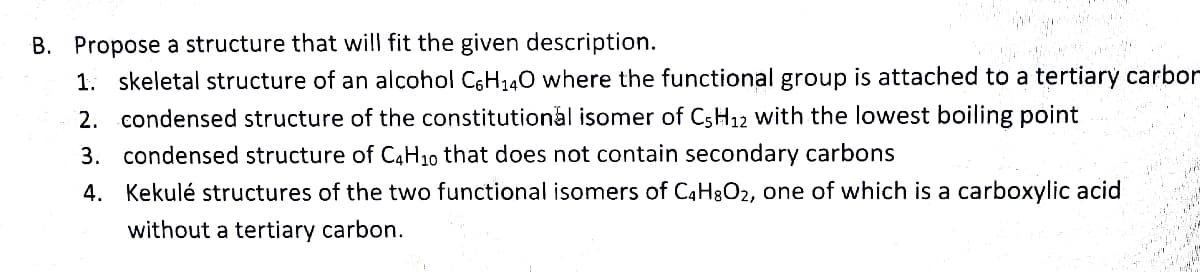
Transcribed Image Text:B. Propose a structure that will fit the given description.
1. skeletal structure of an alcohol C6H₁40 where the functional group is attached to a tertiary carbor
2. condensed structure of the constitutional isomer of C5H12 with the lowest boiling point
3. condensed structure of C4H10 that does not contain secondary carbons
4.
Kekulé structures of the two functional isomers of C4H8O₂, one of which is a carboxylic acid
without a tertiary carbon.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning