
Concept explainers
Consider the following proposed structures for benzene, each of which is consistent with the molecular formula
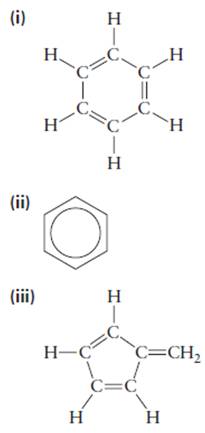
(iv)
(v)
When benzene reacts with chlorine to give
- only one isomer of that compound forms. Which of the five proposed structures for benzene are consistent with this observation? When
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Principles of Modern Chemistry
- Draw the structure of a compound with molecular formula C5H12 that has a. one tertiary carbon. b. no secondary carbons.arrow_forwardDetermine the molecular geometry and polarity of hydroxylamine (NH2OH), formaldehyde (CH2O), and formaldoxime (H2CNOH). Justify your deductions. Is there a difference between reactants and products for both features?.arrow_forwardTrue or false Since the energy of the C-H bond is 416 kj/mol and the energy of the C=0rs bond is 678.5Kj/mol, it can be inferred that methanal is more reactive than methane.arrow_forward
- The carbon–carbon bond length in C2H2 is 1.20 Å, that inC2H4 is 1.34 Å, and that in C2H6 is 1.53 Å. Near which ofthese values would you predict the bond length of C2 tolie? Is the experimentally observed value, 1.31 Å, consistent with your prediction?arrow_forwardDraw structures that meet the following descriptions (there are many possibilities): (a) Three isomers with the formula C8H18 (b) Two isomers with the formula C4H8O2arrow_forwardFormula: C9H10O3 . Determine the structure of the compound given the data shown in the pictures. Explain how you arrived at the answer.arrow_forward
- Provide two constitutional isomers of the formula of C5H12 with no tertiary carbons.arrow_forwardA compound C3H6O has a hydroxyl group but no doublebonds. Write a structural formula consistent with thisinformation.arrow_forwardExplain why the bond dissociation enthalpy of a C-H bond in benzene is significantly greater than that in alkanesarrow_forward
- In the structure of 1‑methylbicyclo[4.3.0]nonane, identify the primary, secondary, tertiary, and quaternary carbons. Key: primary is p, secondary is s, tertiary is t and quarternary is q.arrow_forwardFor which compound containing a heteroatom (an atom other than carbon or hydrogen) does the molecular ion have an even-numbered mass? For which does it have an odd-numbered mass? Q.) A bromoalkane with the molecular formula CnH2n11Brarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




