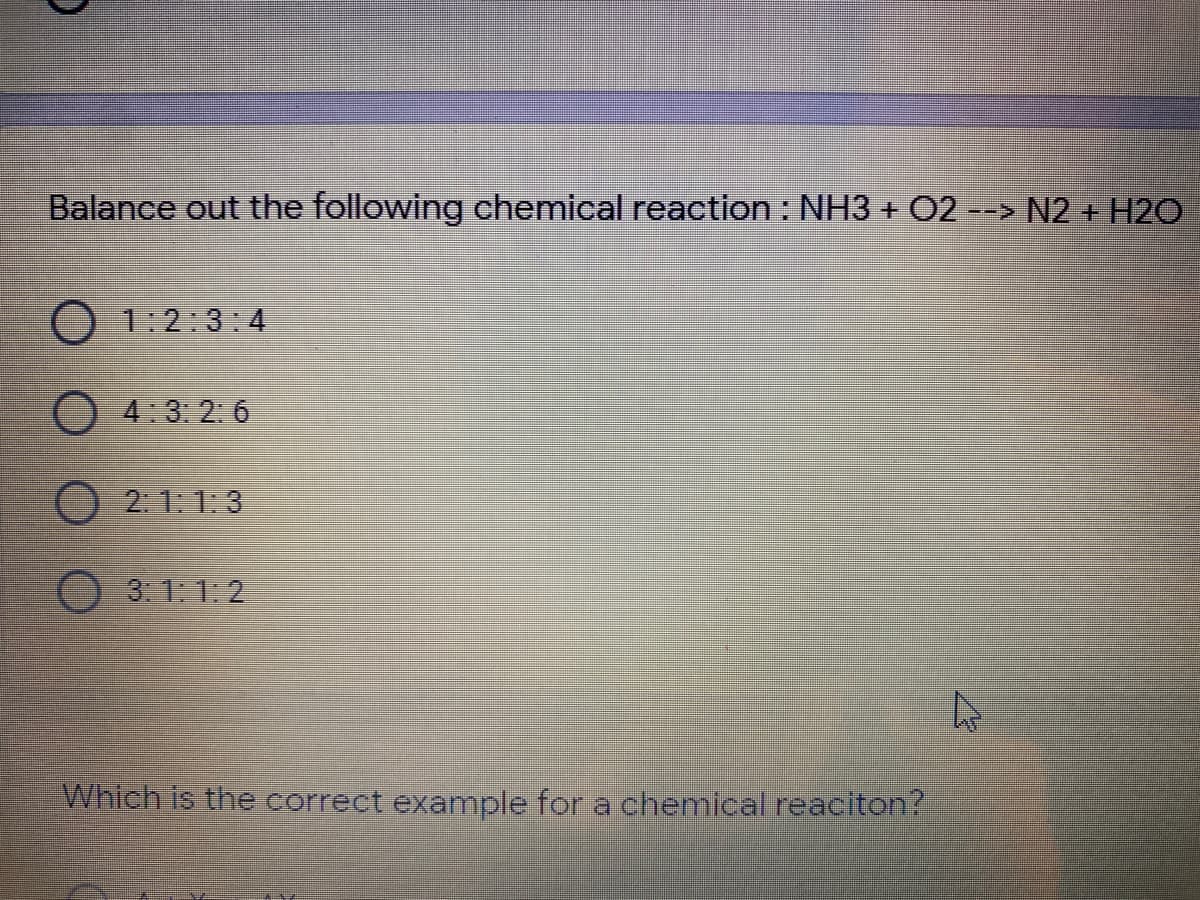
To balance out the following chemical reaction : NH3 + O2 N2 + H2O
Step i) - Start balancing with atoms other than H and O . Count the number of N atoms on both side. There are 2 atoms of N on the product side but only 1 on the reactant side. Multiply N by 2 on the reactant side to balance them .
2NH3 + O2 N2 + H2O
Step ii) - Count the number of H atoms . There are 6 atoms of H on the reactant side and 2 on the product side. Multiply H by 3 on the product side to balance them.
2NH3 + O2 N2 + 3H2O
Step iii) - Next, check O atoms on both sides. There are 2 atoms on the reactant side and three on the product side. the easiest way to balance these is to multiply O2 on the reactant side by 3/2.
2NH3 + 3/2O2 N2 + 3H2O
Balanced equation shouldn't have fractional coefficient, so multiply everything on both sides by 2 to get the final balanced equation.
4NH3 + 3O2 2N2 + 6H2O
Hence, correct option is 4 : 3 : 2 : 6 .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









