Balance the reaction BaCl2 (aq) + AGNO3 (aq) Ba(NO3)2 (aq) + AgCl(s). Express your answer as a chemical equation. Identify all of the phases in your answer.
Balance the reaction BaCl2 (aq) + AGNO3 (aq) Ba(NO3)2 (aq) + AgCl(s). Express your answer as a chemical equation. Identify all of the phases in your answer.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 32QAP: Read the “Chemistry in Focus” segmentConcrete—An Ancient Material Made Newand classify concrete as...
Related questions
Question
What would the balance reaction be?
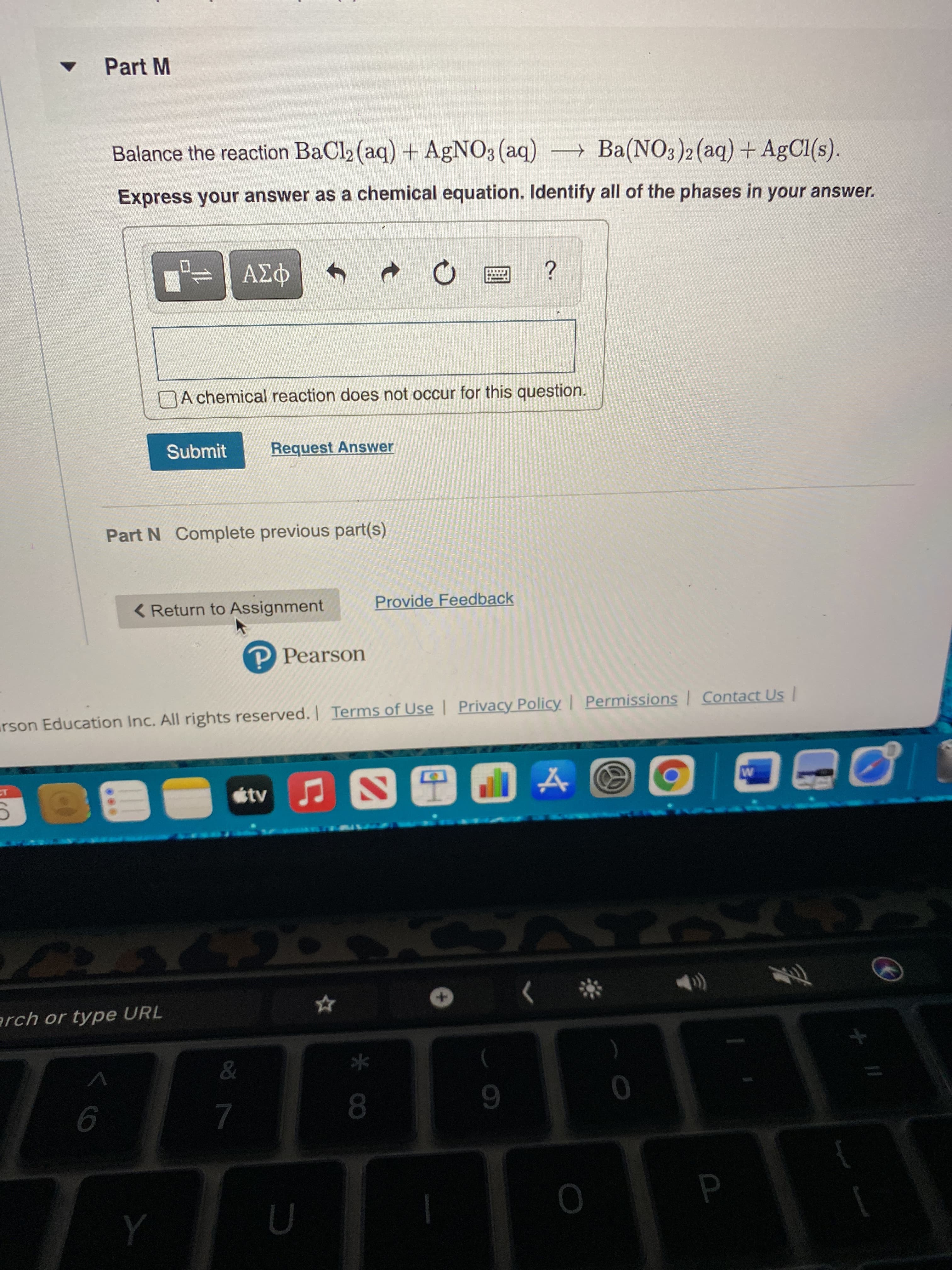
Transcribed Image Text:Part M
Balance the reaction BaCl (aq) + AgNO3(aq) Ba(NO3)2(aq) + AgCl(s).
Express your answer as a chemical equation. Identify all of the phases in your answer.
A chemical reaction does not occur for this question.
Submit
Request Answer
Part N Complete previous part(s)
<Return to Assignment
Provide Feedback
P Pearson
rson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions Contact Us
tvJ
The
erch or type URL
)
08.
6.
9.
7.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning