Cr20;²- (ag) +I(ag)¬Cr* (ag) + IO; (ag) (acidic solution) Express your answer as a chemical equation. Identify all of the phases in your answer.
Cr20;²- (ag) +I(ag)¬Cr* (ag) + IO; (ag) (acidic solution) Express your answer as a chemical equation. Identify all of the phases in your answer.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.57E
Related questions
Question
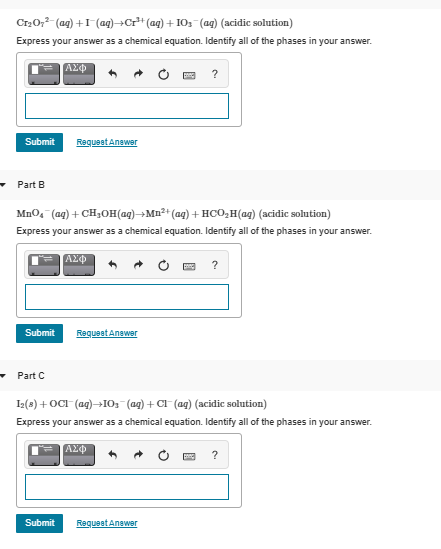
Transcribed Image Text:Cr20;²- (ag) +I(ag)¬Cr* (ag) + IO; (ag) (acidic solution)
Express your answer as a chemical equation. Identify all of the phases in your answer.
Expert Solution
Step 1
Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved then please specify the question number or post only the question.
The half reaction method or ion- electron method is used for balancing a redox reaction. It can be done in acidic or basic medium. In both medium we follow similar steps. The only difference is that in acidic medium we will have hydronium ion and in basic medium we will have hydroxide ion for balancing hydrogen.
Half reaction method in acidic medium involves several steps. They are as follows.
- Separate the half reactions
- Balance all the elements other than oxygen and hydrogen
- Add water to balance oxygen
- Since the medium is acidic add hydronium ion for balancing hydrogen
- Balance charge by adding electron
- Balance no of electrons in each half reaction
- Add both reactions and cancel out common terms
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,



Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

