Q: an esterification reaction, a carboxylic acid chemically reacts with an alcohol to form --- and ---.…
A: In an esterification reaction, a carboxylic acid chemically reacts with an alcohol to form Ester and…
Q: What is the pH of a buffer made from 0.140 mol of HCNO (Ka = 3.5 × 10⁻⁴) and 0.410 mol of NaCNO in…
A:
Q: (1) The observed half life for this reaction when the starting concentration is 0.415 M is s and…
A:
Q: A sample of helium gas has a volume of 6.30 L at a pressure of 825 mmHg and a temperature of 21∘C.…
A: Given: The initial volume, pressure, and temperature of helium gas are 6.30 L, 825 mmHg, and 21 ∘C…
Q: How long would it take for N2O5 to decompose 40%?
A:
Q: The pressure of a sample of gas is measured with an open- ended manometer, shown to the right. The…
A: As per the rules only the first question can be answered:
Q: Draw a structure for the major product in each of the following reactions DBU 'Br
A:
Q: Which of the following describes the reaction: ° Bi → He + 0 TI 81 83 alpha decay reaction O beta…
A:
Q: Draw the molecular orbital diagram for NiMe2(PMe3)3. Be sure to properly label
A:
Q: Which statements are true? Zn2+ is formed from the oxidation of Zn(s). The oxidation number for…
A: Which statements are true?
Q: please probide synthesis for both problems H 2.
A: The alkene undergo oxidation reaction in presence of ozone followed by reduction with Zn/H2O to give…
Q: aluminum chlorate .
A:
Q: Use Le Chatelier's Principle to answer the questions about the following reversible reaction: N2O4…
A: ->According to Le-chatelier principle if equilibrium is disturbed by changing factors or…
Q: percent by mass
A:
Q: complete electronic configuration of Ge.
A:
Q: Barium chloride + sodium carbonate
A: Step by step solution are below
Q: Name this compound.
A:
Q: 18. What is the mass of 20.0 L of gaseous nitrogen dioxide (NO2) at STP? 19. A sample of N2…
A: Concept: STP: The of STP is a temperature of 273 K (0° Celsius or 32° Fahrenheit) and the standard…
Q: 1. Nernst, Gibb's relationship to E° and equilibrium. Everything we've learned so far is…
A:
Q: Which of the following processes has a ΔSsys < 0? A) steam condenses B) methyl alcohol…
A: Entropy is a measure of the randomness of the system. The entropy change in a chemical reaction can…
Q: Use the data below to calculate the bond energy for a C-F bond in kJ/mol. Bond Energy (kJ/mol) C-H…
A: Calculate bond enthalpy of C-F bond ---
Q: Give 5 differences of Configurational isomers and Geometrical isomers
A: Isomer have Same molecular Formula but different arrangement in 3D space
Q: Throughout the course, we have highlighted the terms an ode and cathode. There are differences…
A:
Q: Draw the major organic product(s) of the following reaction. CI CH3OH NaOCH,
A:
Q: Some Radioactive Isotopes Useful in Medical Imaging Mode of Isotope Decay Half-life Use in Medical…
A: Both the parts have been solved in the following step.
Q: 1. Consider the acid-base equilibrium shown below on the left side. Here, the base abstracts a…
A:
Q: 0.8 0.6 0.4 0.2 3000 2000 1000 Wavenumber (cm-1)
A: IR mainly explaines the functional groups that are present in molecules Alcohol: O-H stretching ,…
Q: Which of the following describe(s) the following reaction? Choose all that apply. 235U 94 Sr 130Xe +…
A: Which of the following describe the reaction--
Q: Identify which of the following chemical processes results in an increased in entropy of the system.…
A:
Q: Which of the following characterizes a beta ray? Choose all that apply. O is more penetrating than a…
A: Beta rays are composed of negatively charged particles called beta particles , these are similar to…
Q: Br CHz CH3 OH
A: This reaction will follow SN1 mechanism , which involve formation of carbocation and carbocation…
Q: Example 8: An aqueous solution is 0.907 M Pb(NO3),. Determine the molality of lead (II) nitrate,…
A:
Q: A solution contains 0.0140 M Pb2+(aq) and 0.0140 M Sr2+(aq). If you add SO2−4(aq), what will be the…
A: Ion that has lower value KSP , will precipitate first as its ionic product will overcome the KSP…
Q: A generic salt, AB, has a molar mass of 319 g/mol and a solubility of 8.20 g/L at 25 °C. AB(s)…
A:
Q: Write a balanced nuclear equation for the following: The nuclide bismuth-210 undergoes alpha…
A:
Q: Select the bond that was formed during aldol condensation to yield the following compound. В ОН C O…
A:
Q: 9. Which of the following elements has the largest first ionization energy? A) Bе B) Mg С) Са D) Sr…
A: In this question, all the element present in 2nd group of the periodic table and when we move down…
Q: Some Radioactive Isotopes Useful in Medical Imaging Mode of Decay Use in Medical Imaging Isotope…
A: (1) We have to tell the number of protons and the number of neutrons present on the radioactive…
Q: Provide the missing reagents for each of the following sreactions: CH2 но он CH NH2 но DFocus ENG…
A:
Q: What is the reaction of corn oil and sulfuric acid? What happens in Friedel-Crafts Reaction with…
A: Unsaturated fats will react with sulfuric acid to form C-O-S bonds where carbon from oil will attack…
Q: CH3
A: There are two types of naming in chemistry- 1- IUPAC naming 2- common name
Q: Which of the following methods would be successful in producing the product starting from the given…
A:
Q: Consider the endothermic decomposition reaction of chlorine monofluoride at 1116.8 K. 2 CIF(g) =…
A: 16a. Given, 2ClF(g) ⇌ Cl2(g) + F2(g) Temperature (T) = 1116.8 K Equilibrium constant (K) =6.22 ×…
Q: What amount of heat, in kJ, is required to convert 1.60 g of water at 67.0 °C to 1.60 g of steam at…
A: Given: Mass of water = 1.60 g. Initial temperature of water = 67.0 oC And the final temperature of…
Q: Some Radioactive Isotopes Useful in Medical Imaging Mode of Decay Isotope Half-life Use in Medical…
A: Given that, the initial concentration of the Gallium-67 is C0 = 60.3 mg. The half-life of the…
Q: A student is given a 0.1 M solution of an acid and is asked to determine whether it is a solution of…
A: Given: Concentration of acid = 0.1 M And the pH of the solution = 2.3
Q: 4.The following two molecules undergo stereospecific e product for each reaction. H. CI OH ETOH H3C.…
A:
Q: Average Exposure to Radiation from Common Sources Source Dose (mremlyear) Naturally Occurring…
A: Given: The radiation received in an accident = 5.43 × 10-4 Sv
Q: Aluminum nitrate + potassium bromide
A: We can expect aluminium bromide and potassium nitrate as the displacement products.
Q: A sample of helium gas has a volume of 6.30 L at a pressure of 825 mmHg and a temperature of 21∘C.…
A: Given, Note: 760 mmHg = 1 atm Initially Pressure (P1) = 825 mmHg = 1.08553 atm Volume (V1) = 6.30 L…
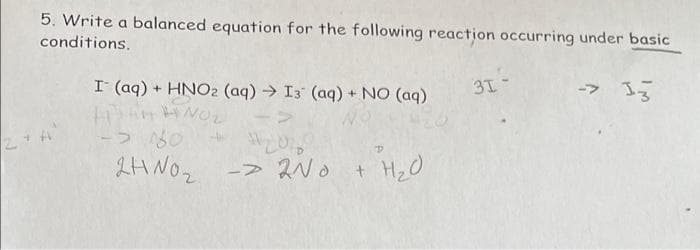
Step by step
Solved in 2 steps

- Sulfuric acid (H2SO4) is called a diprotic acid because it has two acidic protons. The pKa for the first deprotonation is -9, whereas the pKa for the second deprotonation is 2. Explain these relative acid strengths.Two dyes (orange, MW = 300 and blue, MW = 100,000) are placed indialysis tubes and put into beakers of water. Explain why there isorange dye and not blue dye in the beaker waterIf an Ecell of 0.495V was noted when your cell was set-up. What is thesolution pH?
- The following cell was found to have a potential of —0.492 V: Ag|AgCl(sat’d)||HA(0.200 M),NaA(0.300 M)|H2(1.00 atm),Pt Calculate the dissociation constant of HA, neglecting the junction potential.Acetic acid (CH3COOH, abbrev HA) is a very common weak acid with pKa = 4.75 (at 25oC and low ionic strength) A] Write down the dissociation equation of the weak acid HA in aqueous solution, derive the respective formulae for the dissociation constant Ka and pKa. B] Calculate pH of the diluted solution of acetic acid with concentration, c = 0.05 mol/dm3 C] What is the approximate pH of the aqueous acetate buffer solution composed of dissolved mixture of acetic acid (HA) and sodium acetate (NaA) in the same respective concentrations? What is the working pH range of acetate buffers? D] How can you prepare exactly 1 dm3 of acetate buffer solution with required pH 5.0 and the total concentration [HA + NaA], c = 0.01 mol/dm3 if the stock solution of acetic acid (HA) with concentration, cHA = 0.1 mol/dm3, anhydrous sodium acetate (NaA, MT = 82.03 g/mol) and purified water are available? E] Acetate buffer solution may be also prepared by the partial neutralization of HA with NaOH: 20 cm3 of…Question 2. (A) Choose one ionisation technique from the ones discussed in the course (EI, CI, MALDI, ESI) and provide a detailed explanation of how it works, including diagrams if you like. Cover typical applications, and pros/cons, mass analysers that are typically used with this source etc.
- Dihydrogen phosphate, H₂PO₄⁻(aq), undergoes the following acid base equilibria in blood: H₂PO₄⁻(aq) ⇌ H⁺(aq) + HPO₄²⁻(aq)⇌ H⁺(aq) + PO₄³⁻(aq). The pKa values for the first and second steps are 7.2 and 12.4, respectively. The pH of human blood is 7.37 and this is tightly regulated. Which of the following statements most accurately describes the concentrations of the different species for the above reactions in blood. (Hint, think of the titration curves for H₂PO₄⁻(aq) and HPO₄²⁻(aq) and their respective pKa's.) A) [H₂PO₄⁻(aq)] is much greater than [HPO₄²⁻(aq)] and [PO₄³⁻(aq)] B) [HPO₄²⁻(aq)] is much greater than [H₂PO₄⁻(aq)] and [PO₄³⁻(aq)]. C) [H₂PO₄⁻(aq)] and [HPO₄²⁻(aq)] are both present and greater than [PO₄³⁻(aq)]. D) [PO₄³⁻(aq)] is much larger than the other concentrations, [H₂PO₄⁻(aq)] and [HPO₄²⁻(aq)]Calculate (to the nearest 0.1%) the proportion of a dose of ebastine (pKa of conjugate acid = 10.3) that will be ionised at pH 7.7. You do not need to include "%" in your answer.Assign the following as Ror S.
- (9.29 × 105/8)-20.81 = with correct sig figsspectrochemical series which is the interaction relationship of Lewis bases with metals oftransition: a) Explain the order of each base in relation to Fe3+. Why?b) Determine the order of bases with respect to NH4+. Why?2 Two nitroimidazole drugs, misonidazole and tinidazole have single-electron reduction potentials of -0.389 V and -0.464 V respectively: Misonidazole + e- ➝ Misonidazole·- E0 = -0.389 V Tinidazole + e- ➝ Tinidazole·- E0 = -0.464 V The reduction potential of the NADH/NAD couple is -0.324 V, and the reduction potential of the ferrodoxin couple is -0.460 V: NAD+ + e- ➝ NADH E0 = -0.324 V [Ferrodoxin]3+ + e- ➝ [Ferrodoxin]2+ E0 = -0.460 V Explain which, if any, of misonidazole and tinidazole will be selectively reduced in protozoa over mammalian cells. In your answer you should calculate any reduction potentials you need.

