Coding Strand of DNA
When pointing to DNA transcription, the coding strand is found to be the DNA strand whose base sequence is indistinguishable from the base sequence of the RNA transcript developed. It is this strand that comprises the codons, while the non-coding strand comprises the anti-codons.
Nucleotide
Both DNA and RNA are composed of organic molecules known as nucleotides. Hence, nucleotides are known as the basic building blocks of nucleic acids. These substances play a role in various processes such as cell signalling, enzyme reactions, metabolism, and so on.
Structure of Cytosine
Cytosine is among the five primary nitrogenous bases of which DNA and RNA and are being used in storage and transportation of genetic makeup within a cell. Adenine, guanine, thymine as well as uracil are the remaining four nucleobases.
The 2′,3′-cyclic phosphodiester that is formed when RNA is cleaved, forms a mixture of
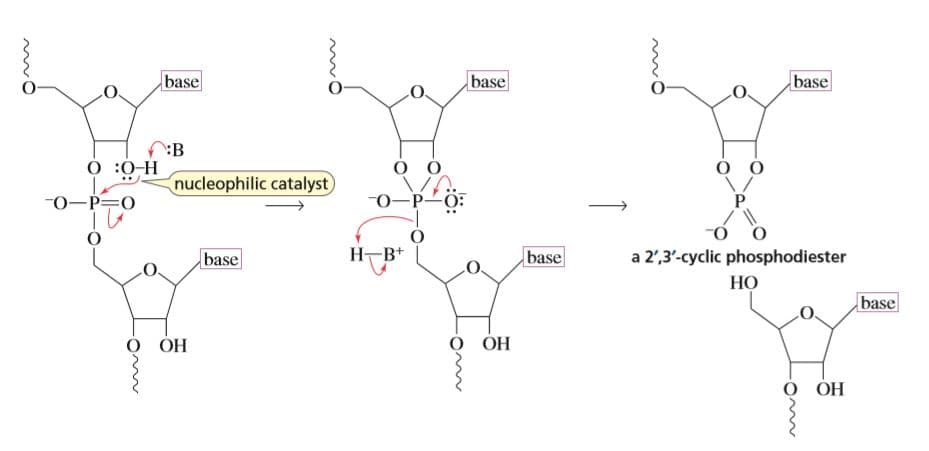
This is the reaction in which the phosphodiester bond in the sugar phosphate of the RNA backbone is cleaved. The hydroxyl group present at the 2' position makes it susceptible for base catalyzed hydrolysis. This is the reason why RNA is less stable when compared to DNA.
There are four steps involved in the mechanism:
1. Deprotonation of the hydroxyl group present on the 2' position:There is a removal of proton from the hydroxyl group due to the catalyst which results in the formation of nucleophile O-.
2. Nucleophilic attack of the deprotonated hydroxyl group on the adjacent phosphorous:The nucleophile thus formed in the first step attacks the phosphorous present adjacent to it.This results in the formation of a transition state.
3. Transition state:This results in the formation of a transition state which is highly unstable.
4. Formation of 2',3'-cyclic phosphodiester which hydrolyzes to give either 2' or 3' phosphate:Since the transition state is unstable it undergoes hydrolysis in the presence of water to give either 2' or 3' phosphate.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images




