Based on the phase diagram below: a. Describe what one would see at pressures and temperatures above 2.0 atm and 450K. b. Describe the phase changes from 50K to 250K at 1.5atm. c. What exists in a system that is at 1 atm and 350K. d. What exists in a system that is at 1 atm and 175K.
Based on the phase diagram below: a. Describe what one would see at pressures and temperatures above 2.0 atm and 450K. b. Describe the phase changes from 50K to 250K at 1.5atm. c. What exists in a system that is at 1 atm and 350K. d. What exists in a system that is at 1 atm and 175K.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.17E
Related questions
Question
Based on the phase diagram below:
a. Describe what one would see at pressures and temperatures above 2.0 atm and 450K.
b. Describe the phase changes from 50K to 250K at 1.5atm.
c. What exists in a system that is at 1 atm and 350K.
d. What exists in a system that is at 1 atm and 175K.
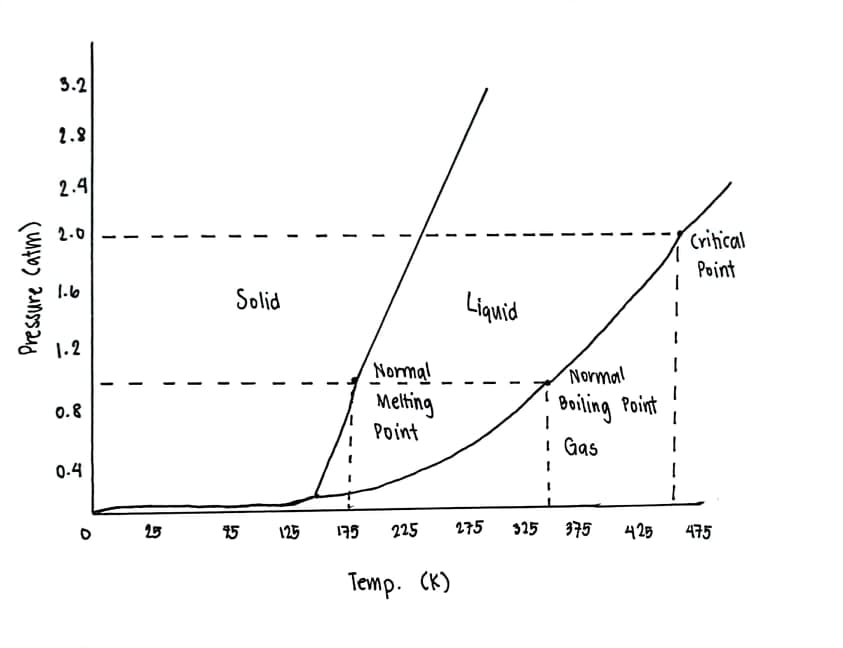
Transcribed Image Text:3.2
2.8
2.4
2.0
Critical
Point
1.6
Solid
Liquid
1.2
Normal
Melting
Normal
Beiling Point
0.8
Point
I Gas
0.4
25
15
125
195
225
275
325 375
425
475
Temp. (K)
Pressure catm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning