b)In magnetron sputtering technique, the pressure of Argon gas 10* Torr. What is the density of the Argon gas? What is the mean free path of the Argon gas? Argon diameter is 3.83Å. Given Data: -23 Boltzmann constant = 1.38 x 10 J/K, 1 Torr = 133 Pa, molecular weight of Argon = 39.96 g/mol.
b)In magnetron sputtering technique, the pressure of Argon gas 10* Torr. What is the density of the Argon gas? What is the mean free path of the Argon gas? Argon diameter is 3.83Å. Given Data: -23 Boltzmann constant = 1.38 x 10 J/K, 1 Torr = 133 Pa, molecular weight of Argon = 39.96 g/mol.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 67E: The compression ratio in an automobile engine is the ratio of the gas pressure at the end of the...
Related questions
Question
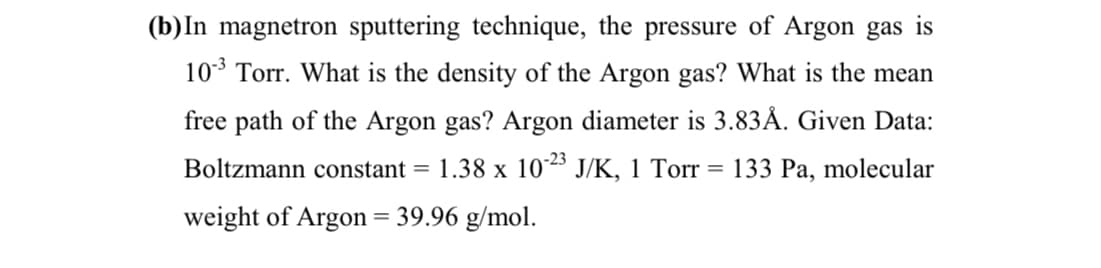
Transcribed Image Text:(b)In magnetron sputtering technique, the pressure of Argon gas is
10* Torr. What is the density of the Argon gas? What is the mean
free path of the Argon gas? Argon diameter is 3.83Å. Given Data:
Boltzmann constant =
1.38 x 1023 J/K, 1 Torr = 133 Pa, molecular
weight of Argon = 39.96 g/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
