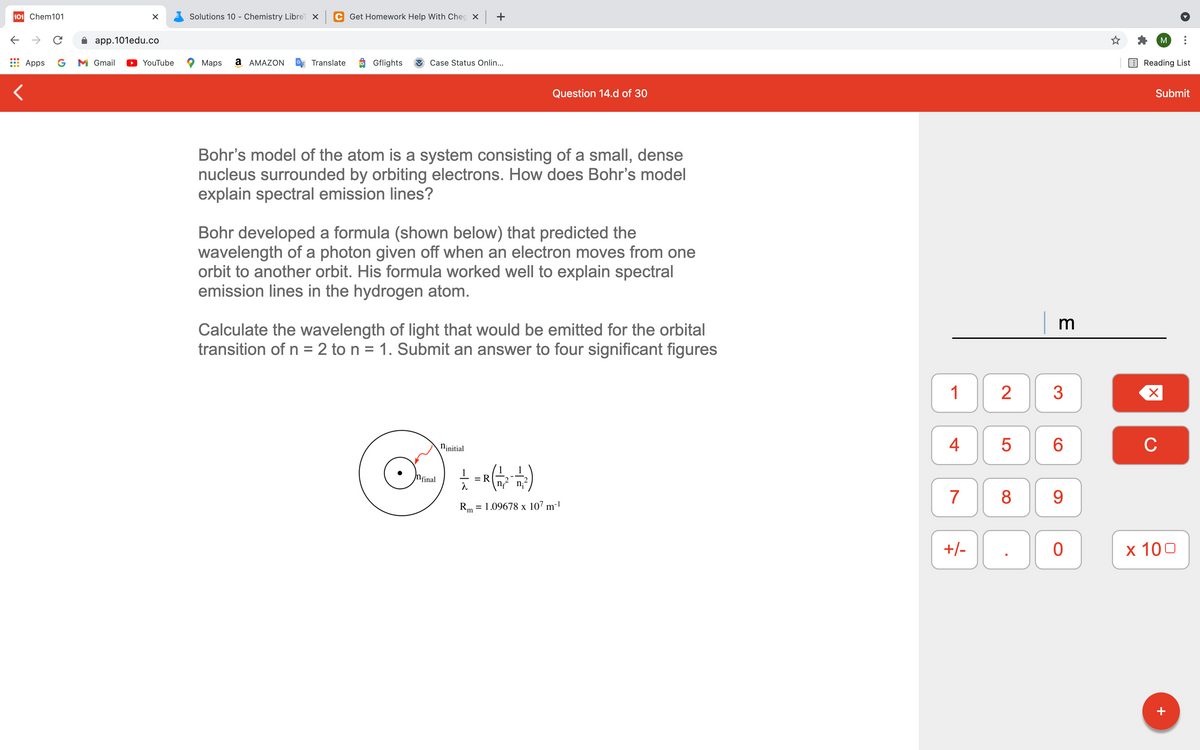
Bohr's model of the atom is a system consisting of a small, dense nucleus surrounded by orbiting electrons. How does Bohr's model explain spectral emission lines? Bohr developed a formula (shown below) that predicted the wavelength of a photon given off when an electron moves from one orbit to another orbit. His formula worked well to explain spectral emission lines in the hydrogen atom. Calculate the wavelength of light that would be emitted for the orbital transition of n = 2 to n = 1. Submit an answer to four significant figures tial R= 1.09678 x 107 m
Bohr's model of the atom is a system consisting of a small, dense nucleus surrounded by orbiting electrons. How does Bohr's model explain spectral emission lines? Bohr developed a formula (shown below) that predicted the wavelength of a photon given off when an electron moves from one orbit to another orbit. His formula worked well to explain spectral emission lines in the hydrogen atom. Calculate the wavelength of light that would be emitted for the orbital transition of n = 2 to n = 1. Submit an answer to four significant figures tial R= 1.09678 x 107 m
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 141QRT
Related questions
Question
Transcribed Image Text:101 Chem101
Solutions 10 - Chemistry LibreT X
C Get Homework Help With Cheg X ++
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
Gflights
Case Status Onlin...
Reading List
Question 14.d of 30
Submit
Bohr's model of the atom is a system consisting of a small, dense
nucleus surrounded by orbiting electrons. How does Bohr's model
explain spectral emission lines?
Bohr developed a formula (shown below) that predicted the
wavelength of a photon given off when an electron moves from one
orbit to another orbit. His formula worked well to explain spectral
emission lines in the hydrogen atom.
| m
Calculate the wavelength of light that would be emitted for the orbital
transition of n = 2 to n = 1. Submit an answer to four significant figures
%3D
1
2
Ninitial
4
C
Nfinal
%3D
7
8
9
Rm = 1.09678 x 107 m-!
+/-
х 100
+
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning