Bromine adds to cis- and trans-2-butene to give different diastereomers of 2,3-dibromo- butane. What does this say about the mode of addition of bromine to this alkene? H3C Br Bry H CH, Br `H. H,C Br Brg HH Br CH3
Bromine adds to cis- and trans-2-butene to give different diastereomers of 2,3-dibromo- butane. What does this say about the mode of addition of bromine to this alkene? H3C Br Bry H CH, Br `H. H,C Br Brg HH Br CH3
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter6: Reactions Of Alkenes
Section: Chapter Questions
Problem 6.17P: Predict the organic product(s) of the reaction of 2-butene with each reagent. (a) H2O (H2SO4) (b)...
Related questions
Question
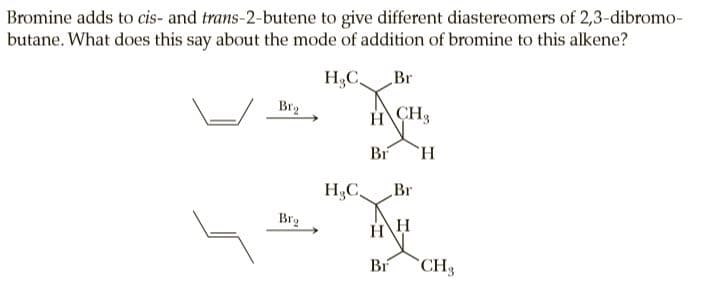
Transcribed Image Text:Bromine adds to cis- and trans-2-butene to give different diastereomers of 2,3-dibromo-
butane. What does this say about the mode of addition of bromine to this alkene?
H3C
Br
Bry
H CH,
Br
`H.
H,C
Br
Brg
HH
Br
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning