(C) Plot the resualts tor Experimenis 1 and 2 on the grid and draw two smooth line graphs. Clearly label your graphs. 40- 30 mperature 10- 10 20 30 40 50 60 volume of acid/ added/ cm d) From your graph, deduce the temperature of the mixture when 6cn3 of acid C reacted with potassium hydroxide in Experiment 1. Show clearly on the graph how you worked out your answer. °C
(C) Plot the resualts tor Experimenis 1 and 2 on the grid and draw two smooth line graphs. Clearly label your graphs. 40- 30 mperature 10- 10 20 30 40 50 60 volume of acid/ added/ cm d) From your graph, deduce the temperature of the mixture when 6cn3 of acid C reacted with potassium hydroxide in Experiment 1. Show clearly on the graph how you worked out your answer. °C
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
Pls do the d part which on bottom of the page by referring the graph, the question consists of science
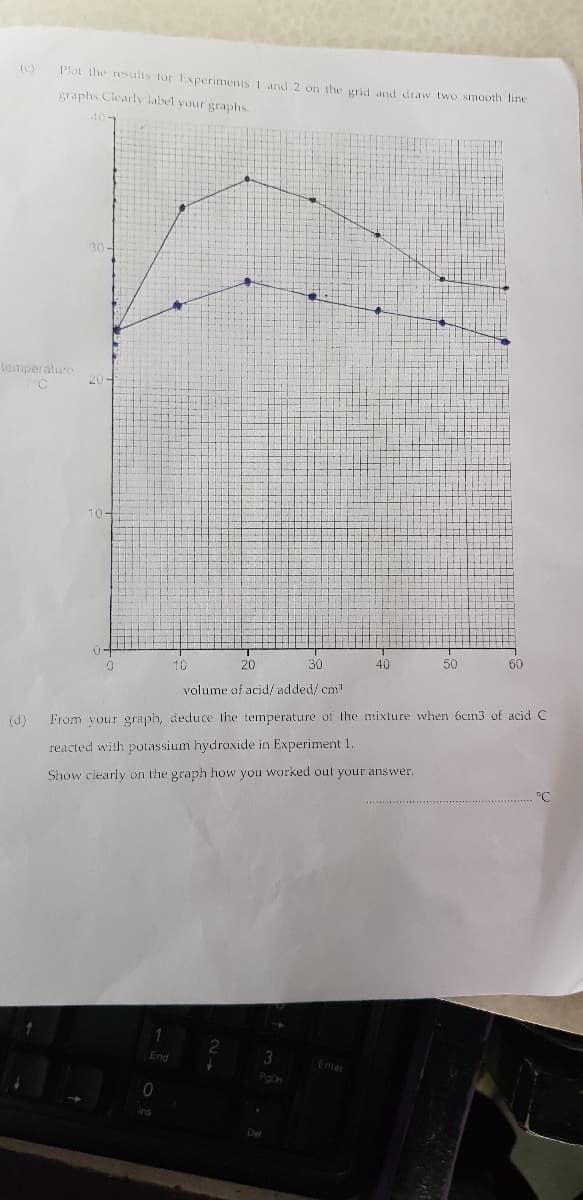
Transcribed Image Text:(c)
Plot the results tor Experimenis 1 and 2 on the grid and draw two smooth line
graphs.Clearly label your graphs.
30-
temperature
10-
10
20
30
40
50
60
volume of acid/ added/ cm
(d)
From vour graph, deduce the temperature of the mixture when 6cm3 of acid C
reacted with potassium hydroxide in Experiment 1.
Show clearly on the graph how you worked out your answer.
°C
Enter
Det
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
