The density of aluminum is 2.70 g/cm'. Calculate the thickness of a rectangular sheet of aluminum foil with a width of 11.5 cm, a length of 14.0 cm, and a mass of 2.04 g. -Examine your results from your data table in Part 3. Do you have any values for the density of the salt solution that lie OUTSIDE the range (& 2s)? If so, list them here: Recalculate & by omitting values that lie OUTSIDE the range. This is the density value you should use to determine your experimental % NaCl. part 3 data table included
The density of aluminum is 2.70 g/cm'. Calculate the thickness of a rectangular sheet of aluminum foil with a width of 11.5 cm, a length of 14.0 cm, and a mass of 2.04 g. -Examine your results from your data table in Part 3. Do you have any values for the density of the salt solution that lie OUTSIDE the range (& 2s)? If so, list them here: Recalculate & by omitting values that lie OUTSIDE the range. This is the density value you should use to determine your experimental % NaCl. part 3 data table included
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 13Q
Related questions
Question
-The density of aluminum is 2.70 g/cm'. Calculate the thickness of a rectangular sheet
of aluminum foil with a width of 11.5 cm, a length of 14.0 cm, and a mass of 2.04 g.
-Examine your results from your data table in Part 3. Do you have any values for the
density of the salt solution that lie OUTSIDE the range (& 2s)? If so, list them here:
Recalculate & by omitting values that lie OUTSIDE the range. This is the density value
you should use to determine your experimental % NaCl.
part 3 data table included
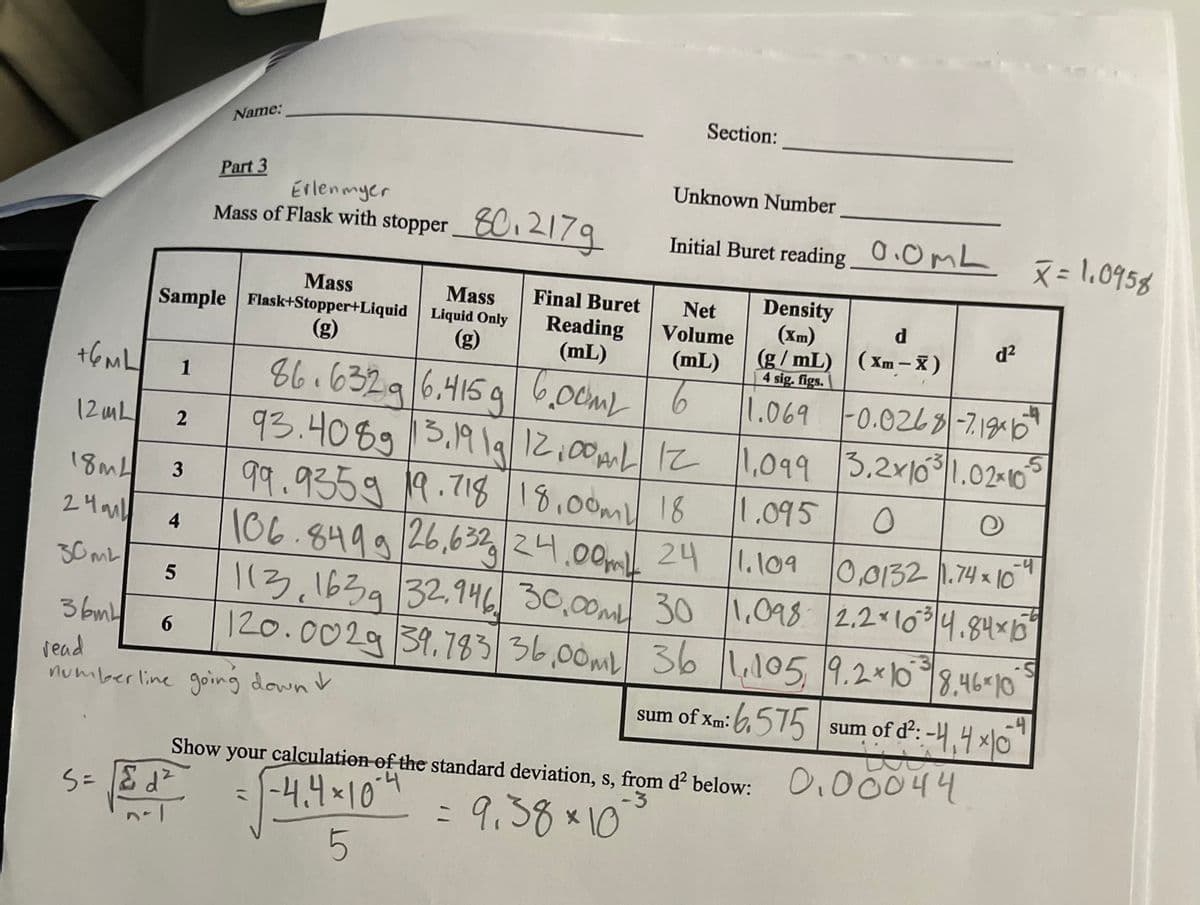
Transcribed Image Text:+6ML
12mL
18mL
24ml
30ML
36mL
read
Mass
Sample Flask+Stopper+Liquid
(g)
2
3
4
5
6
1
Name:
Part 3
Erlenmyer
Mass of Flask with stopper 80.217g
S=&d²
Final Buret
Reading
(mL)
86.6329 6.415g 6.00ML
numberline going down &
Mass
Liquid Only
(g)
Section:
= √-4₁
Unknown Number
Initial Buret reading
Net Density
Volume
(mL)
6
sum of Xm:
Show your calculation of the standard deviation, s, from d² below:
-4.4 × 10-4
-3
= 9.38×10
5
0.0mL
93.4089 13.19 19 12.00 12
99.9359 19.718 18.00m 18
1,099
1.095
O
e
-4
106.8499 26.632 24.00m 24 1.109 0.0132 1.74 x 10
113, 1639 32.946 30,00ML 30 1,098 2.2 × 10³ 4.84x1535
120.0029 39.783 36,00ML 36 1.105 9.2×10 8.46 10
-S
6.575
-4
d
(g/mL) (Xm-X)
4 sig. figs.
1.069 -0.0268-7.186²
3.2x1031.02-10
d²
X = 1.0958
sum of d²: -4,4x10
ti
0.00044
-5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning