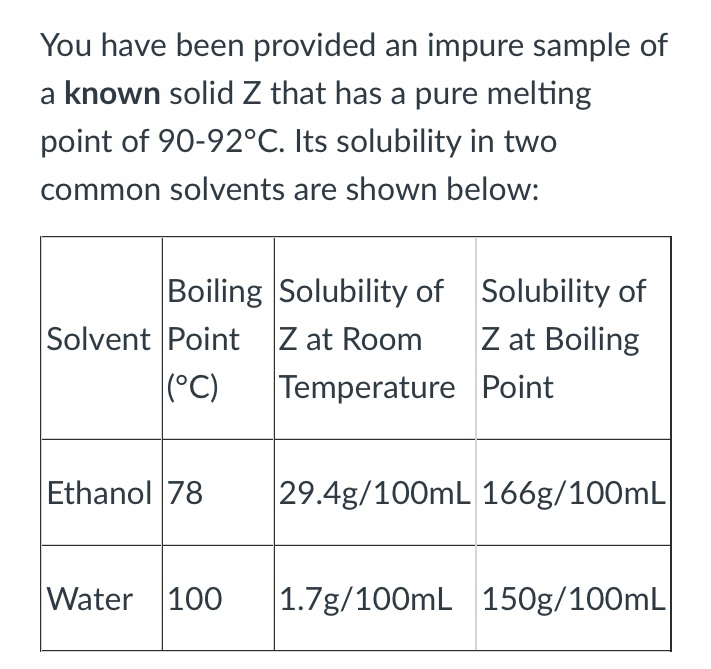
c) You decide to use ethanol in conjunction with another solvent to make a solvent pair. What two criteria does this second solvent have to have in order to form a good solvent pair with ethanol for solid Z?
Q: The liquid-liquid extraction is the classical technique in chemistry to isolate a target component…
A: Liquid-liquid extraction is also known as solvent extraction in which compounds are separated based…
Q: A mixture is 10.0 mole% Methyl alcohol, 75.0 mole% ethyl acetate, and 15.0 mole% acetic acid.…
A: Number of moles of a compound is equal to the ratio of given weight of the compound to the molecular…
Q: Brian forgot to close the end of his capillary tube when he was conducting the boiling point…
A: The correct option is 'a', a. Brian did a great job in making sure that the experiment worked. It is…
Q: Why should the recrystallization solvent have a fairly low boiling point?
A:
Q: If you are extracting a substance from water into ether, is it more effective to do one extraction…
A:
Q: For a two-solvent recrystallization, you should have one solvent (solvent # 1) in which your desired…
A: In the process of crystallization first the solid compound is dissolved (In solvent 1) at boiling…
Q: If you do not heat your crucible strongly enough to remove all volatile impurities when you heat the…
A: The loss of water happening during the inital heating or during the second heating is not relevant
Q: Imagine your original mixture had contained a component capable of sublimation, such as naphthalene.…
A:
Q: What is each cycle of a simple distillation called? Theoretical plate Distillate…
A: Simple distillation is process of separation.
Q: The key to a successful HPLC procedure is good sample separation which is represented by a clean…
A: In this question, we have to choose the correct option from the given options.
Q: i) What two criteria should you ensure about your melting point sample before you fill the melting…
A: Since you have posted multiple questions, but we will solve first question for you. For remaining…
Q: 3. Rank these solvent systems from on that would cause a compound to elute from the column fastest…
A: Given : Mixture of solvents To find : Solvent that elute the Compound fastest and slowest .…
Q: It is tempting to maximize the amount of product isolated by continuing the fractional distillation…
A: The solution is given below -
Q: - What is the ideal solvent behavior for crystallization? - You will probably hear me say…
A: Crystallization is defined as the process in which atoms in a given sample are arranged into a…
Q: Why is it not necessary to dry the Erlenmeyer flask between each trial?
A:
Q: (a) Suppose that CaO is added as an impurity to Li2O. If the Ca2+ substitutes for Li+ , what kind of…
A:
Q: How can a fractionating column be prepared from a standard distillation column? Select one: Pack…
A: The fractionating column is fitted above the distillation flask containing the mixture of liquids to…
Q: Isopropanol is a good extraction solvent for plant pigments as it breaks down cell walls and is…
A: Spinach leaves has few more pigments other than chlorophyll-a, that are carotenes, chlorophyll-b and…
Q: Is Column 2 (greater than, less than, equal to, cannot be determined) with Column 4 5. Given: Cr|…
A:
Q: 1. How can one get pure ethanol from the azeotropic mixture? (This extra process explains why…
A: Azeotrope are difficult to separate due to high molecular interaction. Their molecular…
Q: When should we stop collecting distillate in ethanol-alcohol simple distillation process? Give me…
A: We are find out when to stop collecting distillate in ethanol distillation process.
Q: What effect would the incomplete drying of a sample (e.g., the incomplete removal of a…
A: The melting point will be lowered and broadened.
Q: 3. What are su melting point using a capillary and a Thiele tube containing 4. Is it possible for a…
A:
Q: Why was there a change to the way the dye molecules partition between the two solvents when ethanol…
A: 2)
Q: What would happen if the Erlenmeyer flask containing the crude dba in EtOH undergoing…
A: As we know during recrystallisation we dissolve our product into suitable solvent such as here, dba…
Q: 4. What is the purpose of changing the eluting solvent from pentane to ether between the two…
A: "Since there are multiple questions and it is not mentioned that which one has to be solved so I am…
Q: The question is: Consider a mixture containing 0.5 benzil and 0.05 biphenyl. Your task is to…
A: Extraction:It is a process used for the separation of a substance of interest mixed with others.…
Q: Distillation Curve 110 100 90 80 70 60 50 40 20 40 60 100 120 Volume (drops) 13. Based from the…
A: Distillation is a technique in which the mixture of liquid can be separated.
Q: d) Describe the procedure you would use to dissolve solid Z in a solvent pair of ethanol and this…
A: Both the solution are given below.
Q: What is not typically combined in equal quantities in a Mixed Melting Point? The unknown and the…
A: At the time of distillation, compounds are seperated on the basis of their melting point.
Q: What is the approximate amount (mL) of 95% ethyl alcohol that will be required to dissolve the…
A: Given: Solubility of sulfanilamide in ethanol is: 14 mg at 0 0C 24mg at 20 0C 46mg at 40 0C 88mg at…
Q: Why was n-octanol chosen as a surrogate for natural organic phases? Why not another solvent such as…
A: A question based on introduction to organic chemistry that is to be accomplished.
Q: (i) Name any fraction, which is obtained higher up the distillation column than gas oil, and give…
A: The answer is attached-:
Q: Why is there a change to the way the dye molecules partition between two solvents when ethanol is…
A:
Q: In this lab, you used rotary evaporation to remove the neutral organic solvent. Rotary evaporation…
A: A rotary evaporator is a device used in chemical laboratories for the efficient and gentle removal…
Q: In extraction process, the solution contains mixture of benzoic acid and sodium chloride and was…
A: Extraction process is a type of process in which when a reaction is completed, sometimes instead of…
Q: you answer and explain this? a. Explain in your own words how the concept of vapor pressures would…
A: Answer Steam Distillation. Steam distillation is a separation method that involves distilling water…
Q: One of the components of nutmeg is an organic compound, Trimiristin, A. To extract this compound…
A: One of the components of nutmeg is an organic compound, Trimiristin, A. To extract this compound…
Q: 5. For the same solid compound as the question above, if the 1 mm sample was heated at 60° per…
A: (4) Heating rate = Temperature/time Heating rate = 200 oC/1 oC/min Heating rate = 200 min (5) 1 mm…
Q: Which compound(s) are more soluble in water than ligroin? Select all that apply. toluene oxalic…
A: We know that, according to the solubility rules, like dissolves like. I.e we can say that, a polar…
Q: Please answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. 1-butanol, 2-butanol, and…
A: "Like dissolves like" is the thumb rule for dissolution of solute in solvent. This means highly…
Q: 2) determine the identity of an unknown solid. Briefly explain why the experimental technique of…
A: The Temperature at which the solid state compound transform into liquid state without changing its…
Q: Filter paper is usually a poor material on which to powder a solid sample before introducing it…
A: Filter paper is not used as material on which substance can be powdered before its addition into…
Q: Why is it dangerous to attempt a distillation in a completely closed system
A: Distillation : a process of separating mixture of compounds based on difference in their boiling…
Q: Which combination cannot be used as mobile phase in RPLC? A. Acetonitrile-cyclopentane B.…
A: Which combination cannot be used as mobile phase in RPLC? answer is given below with explanation.
Q: 1. Why does the solid need to be finely ground and then tightly packed in a melting point capillary…
A: The temperature at which the solid melts and starts converting into liquid is called as melting…
Q: Based on the resulting chromatograms after visualization with iodine vapor, which between the…
A: A question based on purification, which is to be accomplished.
Q: for benzoic acid: a.) If a benzoic acid sample had colored impurities, the solvent to choose would…
A: Decolorizing carbon provide a large surface area to which large colored molecules may become…
help

Step by step
Solved in 3 steps

- In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg. How many moles of solute is present in the solution? Final answer must be rounded off to 2 decimal places, and shall NOT have any units.In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg. 1. How many moles of solute is present in the solution 2. What is the value for delta Tb or the change in boiling temperature (in degrees Celsius)? 3. What is the molal concentration of the solution?In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg. How many moles of solute is present in the solution? Molality of the solution?
- In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg What is the molal concentration of the solution?In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg What is the molal concentration of the solution? Final answer must be rounded off to 2 decimal places, and shall NOT have any unit.In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg. How many moles of solute is present in the solution?
- In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kgIn the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg. How many moles of solute is present in the solution given?In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 degC. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 degC. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg Questions: 1. What is the molal concentration of the solution? 2. What is the value for delta Tb or the change in boiling temperature (in degrees Celsius)? 3. How many moles of solute are present in the solution?
- In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 deg C. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 deg C. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kgIn the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 deg C. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 deg C. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg What is the value for delta Tb or the change in boiling temperature (in degrees Celsius)?In the determination of molar mass of an unknown substance by ebullioscopic constant, 30 mL of acetone (C3H6O) was placed in a test tube with thermometer and glass tubing and subjected to water bath. Upon boiling, the temperature reads 56 deg C. For the boiling point of unknown-acetone solution, you prepared the solution by mixing 1.60 g of unknown solute in the 30 mL acetone and subjected it again to water bath. The boiling temperature of the solution is 56.68 deg C. The density of acetone = 0.9849 g/mL and the Kb of acetone = 1.67 degC/kg What is the value for delta Tb or the change in boiling temperature (in degrees Celsius)? Final answer must be rounded off to 2 decimal places, and shall NOT have any unit.

