C. COMPARING FUELS: A student wanted to compare the energy released when different fuels burned. To make it a fair test she put 1 g of each fuel in a little dish. Then she burned each fuel under calorimeter containing 200 cm³ of water. These are the result. (Complete the table) Fuel Temperature at the start in °C Temperature at the end in °C Temperature rises in °C Methanol 25 56 ethanol 18 53 propanol 17 54 butanol 23 63 A. Calculate each temperature rise and state which fuel releases most energy per gram. Another student used spirit burners for the experiment. She had to weight them before and after each test to find out how much fuel she had used. To make it a fair test she used each fuel to make the same amount of water 10 °C. These are the results. Fuel Mass of burner at the start in g Mass of fuel using g Mass of burner at the end in g 150.7 Methanol 153.3 ethanol 213.4 210.6 propanol 185.8 183.4 butanol 198.5 196.3 B. Calculate the mass of each fuel she had to use to release the same amount of energy, and state which fuel must release more energy per gram.. C. What could you do to prove that your result is repeatable? D. Results are reproducible if they lead to the same conclusions when different people do the experiments or when different methods are used. Are the results reported reproducible?
C. COMPARING FUELS: A student wanted to compare the energy released when different fuels burned. To make it a fair test she put 1 g of each fuel in a little dish. Then she burned each fuel under calorimeter containing 200 cm³ of water. These are the result. (Complete the table) Fuel Temperature at the start in °C Temperature at the end in °C Temperature rises in °C Methanol 25 56 ethanol 18 53 propanol 17 54 butanol 23 63 A. Calculate each temperature rise and state which fuel releases most energy per gram. Another student used spirit burners for the experiment. She had to weight them before and after each test to find out how much fuel she had used. To make it a fair test she used each fuel to make the same amount of water 10 °C. These are the results. Fuel Mass of burner at the start in g Mass of fuel using g Mass of burner at the end in g 150.7 Methanol 153.3 ethanol 213.4 210.6 propanol 185.8 183.4 butanol 198.5 196.3 B. Calculate the mass of each fuel she had to use to release the same amount of energy, and state which fuel must release more energy per gram.. C. What could you do to prove that your result is repeatable? D. Results are reproducible if they lead to the same conclusions when different people do the experiments or when different methods are used. Are the results reported reproducible?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 14QAP: In earlier times, ethyl ether was commonly used as an anesthetic. It is, however, highly flammable....
Related questions
Question
Complete the table and answer the question in letter b,c and d.
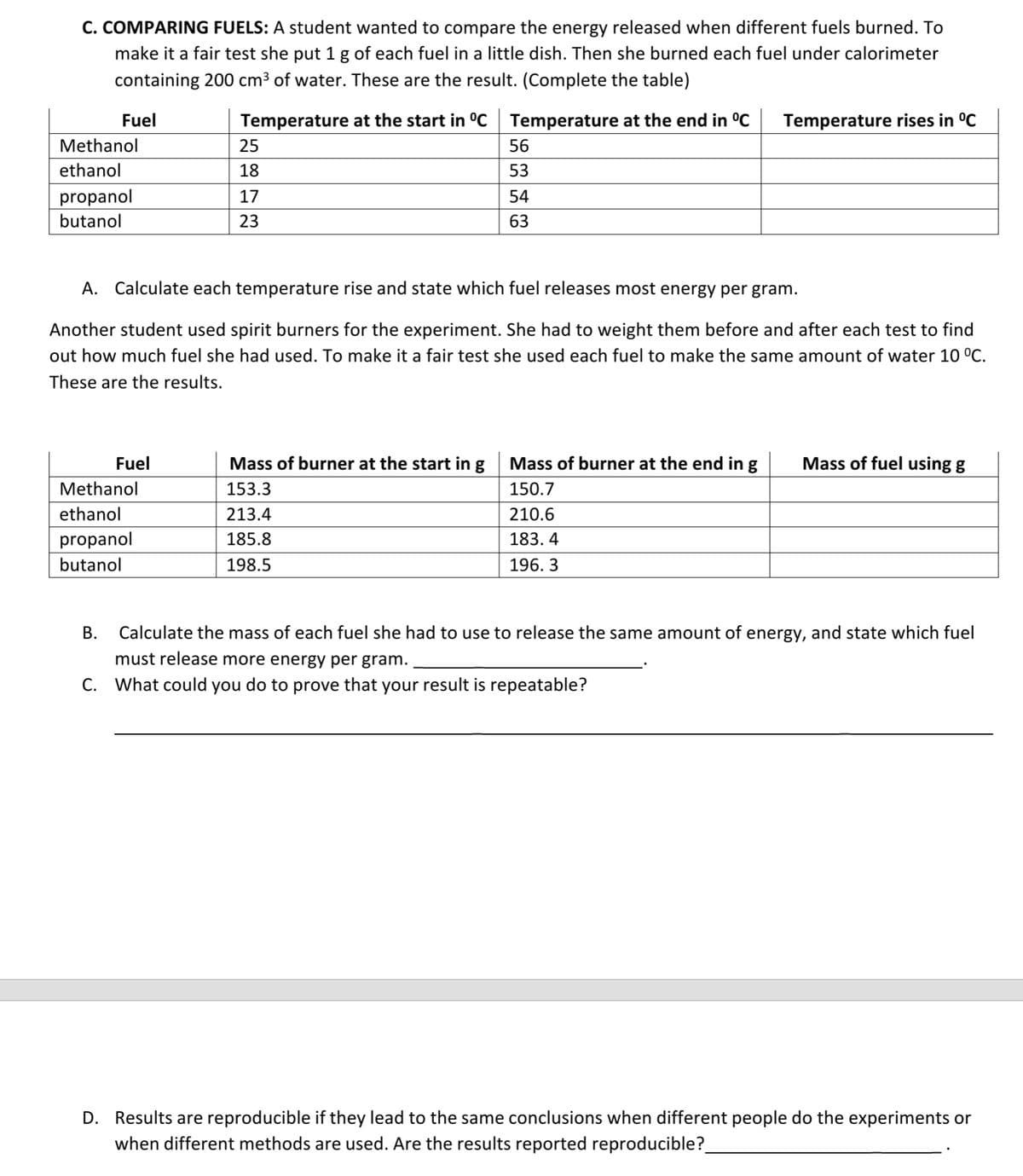
Transcribed Image Text:C. COMPARING FUELS: A student wanted to compare the energy released when different fuels burned. To
make it a fair test she put 1 g of each fuel in a little dish. Then she burned each fuel under calorimeter
containing 200 cm³ of water. These are the result. (Complete the table)
Fuel
Temperature rises in °C
Temperature at the start in °C Temperature at the end in °C
25
Methanol
56
ethanol
18
53
propanol
17
54
butanol
23
63
A. Calculate each temperature rise and state which fuel releases most energy per gram.
Another student used spirit burners for the experiment. She had to weight them before and after each test to find
out how much fuel she had used. To make it a fair test she used each fuel to make the same amount of water 10 °C.
These are the results.
Fuel
Mass of fuel using g
Mass of burner at the start in g
153.3
Mass of burner at the end in g
150.7
Methanol
ethanol
213.4
210.6
propanol
185.8
183.4
butanol
198.5
196.3
B.
Calculate the mass of each fuel she had to use to release the same amount of energy, and state which fuel
must release more energy per gram.
C. What could you do to prove that your result is repeatable?
D. Results are reproducible if they lead to the same conclusions when different people do the experiments or
when different methods are used. Are the results reported reproducible?_
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax