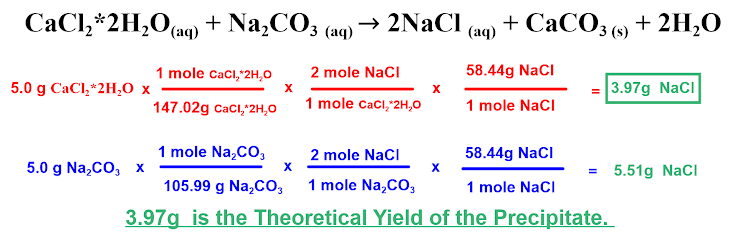
CaCl,*2H,O(aq) + Na,CO3 (a4) → 2NaCl + CaCO3 () + 2H,0 (aq) 1 mole Cacl,"2H,O 2 mole NacI 58.44g NaCI 5.0 g CaCl,*2H,O x 3.97g Naci 147.02g cacı,"2H,0 1 mole cacı,"2H,0 1 mole Naci 1 mole Na,CO, 58.44g Naci X 2 mole Naci 5.0 g Na,Co, x = 5.51g Naci 105.99 g Na,CO, 1 mole Na,Co, 1 mole Naci 3.97g is the Theoretical Yield of the Precipitate.
CaCl,*2H,O(aq) + Na,CO3 (a4) → 2NaCl + CaCO3 () + 2H,0 (aq) 1 mole Cacl,"2H,O 2 mole NacI 58.44g NaCI 5.0 g CaCl,*2H,O x 3.97g Naci 147.02g cacı,"2H,0 1 mole cacı,"2H,0 1 mole Naci 1 mole Na,CO, 58.44g Naci X 2 mole Naci 5.0 g Na,Co, x = 5.51g Naci 105.99 g Na,CO, 1 mole Na,Co, 1 mole Naci 3.97g is the Theoretical Yield of the Precipitate.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 6PS: A 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of...
Related questions
Question
Do you agree with the work above that the Theoretical Yield of the precipitate is 3.97g?
A) Yes, but I would need to know the Actual Yield to know how close we got to the correct answer
B) No, NaCl is not the precipitate so this does not give you an accurate Theoretical Yield of the precipitate
C) No, the molar mass for Calcium Chloride Dihydrate is 110.98g
D) Yes, 3.97 g is the correct Theoretical Yield of the Precipitate
E) No, 5.51 g is the correct Theoretical Yield of the Precipitate
Transcribed Image Text:CaCl,*2H,O(aq) + Na,CO3 (a4) → 2NaCl
+ CaCO3 () + 2H,0
(aq)
1 mole Cacl,"2H,O
2 mole NacI
58.44g NaCI
5.0 g CaCl,*2H,O x
3.97g Naci
147.02g cacı,"2H,0
1 mole cacı,"2H,0
1 mole Naci
1 mole Na,CO,
58.44g Naci
X
2 mole Naci
5.0 g Na,Co, x
= 5.51g Naci
105.99 g Na,CO, 1 mole Na,Co,
1 mole Naci
3.97g is the Theoretical Yield of the Precipitate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning