Potassium chromate is slowly added to a solution containing 0.10 MAGNO, and 0.10 M Ba(NO,), What is the Ag+ concentration (in mol/L) when BaCro, just starts to precipitate? The Ka, for Ag, Cro and BaCro, are1.1 x 1012 and 1.2 × 1010, respectively. Express your answer in scientific notation rounded to three significant figures.
Potassium chromate is slowly added to a solution containing 0.10 MAGNO, and 0.10 M Ba(NO,), What is the Ag+ concentration (in mol/L) when BaCro, just starts to precipitate? The Ka, for Ag, Cro and BaCro, are1.1 x 1012 and 1.2 × 1010, respectively. Express your answer in scientific notation rounded to three significant figures.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 115E: A volume of 50 mL of 1.8 M NH3 is mixed with an equal volume of a solution containing 0.95 g of...
Related questions
Question
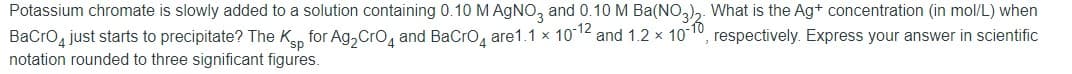
Transcribed Image Text:Potassium chromate is slowly added to a solution containing 0.10 M AGNO, and 0.10 M Ba(NO,),. What is the Ag+ concentration (in mol/L) when
BaCro just starts to precipitate? The Kan for Ag, Cro, and BaCro, are1.1 x 1012 and 1.2 x 10
notation rounded to three significant figures.
respectively. Express your answer in scientific
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
