Caffeine, benzoate, and aspartame content of mountain dew soda was determined using reverse phase HPLC. The concentration of the standard used are as follows, caffeine: 0.70 mg/mL, benzoate: 1.6 mg/mL, and aspartame 5.0 mg/mL. The following volume of the standards were taken to a 50.0 mL volumetric flask and diluted to the mark to construct a calibration curve: Caffeine Aspartame Benzoate 1mL 1 mL 1 mL 2 mL 2mL 2 mL 3 mL 3 mL 3 mL 4 mL 4 mL 4 mL 5 mL 5 mL 5 mL The 12-oz can mountain dew sample were treated as follows: The 12-oz can were has been left out overnight to get rid the carbonation then, 2 mL were drawn into a plastic syringe, filtered, and placed into a vial. An equal amount of deionized water was added. A 100 µL sample were injected into a sample loop using same parameters with the standards. Assume 12 oz - 354.9 ml The following data were obtained: Peak Area Caffeine Benzoate Aspartame Std 1 2.30 3.54 3.33 Std 2 4.10 5.59 5.83 Std 3 7.37 9.98 9.63 Std 4 9.56 14.60 12.78 Std 5 12.18 18.16 16.33 Mountain Dew 10.21 14.97 7.96 Determine the equation of the line for aspartame
Caffeine, benzoate, and aspartame content of mountain dew soda was determined using reverse phase HPLC. The concentration of the standard used are as follows, caffeine: 0.70 mg/mL, benzoate: 1.6 mg/mL, and aspartame 5.0 mg/mL. The following volume of the standards were taken to a 50.0 mL volumetric flask and diluted to the mark to construct a calibration curve: Caffeine Aspartame Benzoate 1mL 1 mL 1 mL 2 mL 2mL 2 mL 3 mL 3 mL 3 mL 4 mL 4 mL 4 mL 5 mL 5 mL 5 mL The 12-oz can mountain dew sample were treated as follows: The 12-oz can were has been left out overnight to get rid the carbonation then, 2 mL were drawn into a plastic syringe, filtered, and placed into a vial. An equal amount of deionized water was added. A 100 µL sample were injected into a sample loop using same parameters with the standards. Assume 12 oz - 354.9 ml The following data were obtained: Peak Area Caffeine Benzoate Aspartame Std 1 2.30 3.54 3.33 Std 2 4.10 5.59 5.83 Std 3 7.37 9.98 9.63 Std 4 9.56 14.60 12.78 Std 5 12.18 18.16 16.33 Mountain Dew 10.21 14.97 7.96 Determine the equation of the line for aspartame
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.25QAP
Related questions
Question
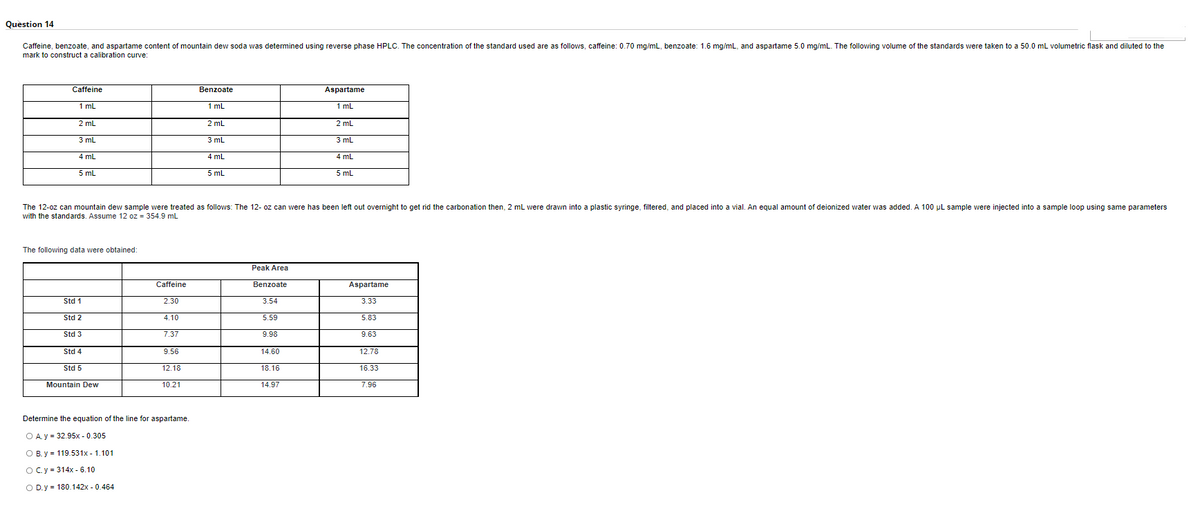
Transcribed Image Text:Question 14
Caffeine, benzoate, and aspartame content of mountain dew soda was determined using reverse phase HPLC. The concentration of the standard used are as follows, caffeine: 0.70 mg/mL, benzoate: 1.6 mg/mL, and aspartame 5.0 mg/mL. The following volume of the standards were taken to a 50.0 mL volumetric flask and diluted the
mark to construct a calibration curve:
Caffeine
Benzoate
Aspartame
1 mL
1 mL
1 mL
2 mL
2 mL
2 mL
3 mL
3 mL
3 mL
4 mL
4 mL
4 mL
5 mL
5 mL
5 mL
The 12-oz can mountain dew sample were treated as follows: The 12- oz can were has been left out overnight to get rid the carbonation then, 2 mL were drawn into a plastic syringe, filtered, and placed into a vial. An equal amount of deionized water was added. A 100 μL sample were injected into a sample loop using same parameters
with the standards. Assume 12 oz = 354.9 mL
The following data were obtained:
Caffeine
Peak Area
Benzoate
3.54
Aspartame
Std 1
2.30
3.33
Std 2
4.10
5.59
5.83
Std 3
7.37
9.98
9.63
Std 4
9.56
14.60
12.78
Std 5
12.18
18.16
16.33
Mountain Dew
10.21
14.97
7.96
Determine the equation of the line for aspartame.
O A.y = 32.95x -0.305
O B. y = 119.531x - 1.101
O C. y = 314x - 6.10
O D.y = 180.142x -0.464
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

