Calcium metal reacts with acid in solution according to the following reaction: Ca(s) + 2H†(aq) → Ca²+ (aq) + H2(g) When 0.1437 g of Ca(s) is combined with enough acid solution to make 150.0 g of solution in a coffee-cup calorimeter, all of the calcium reacts, raising the temperature from 21.80 °C to 24.90 °C. Assume that the solution is mostly water, so that the heat capacity is 4.184 J · g¬1.°C-1.
Calcium metal reacts with acid in solution according to the following reaction: Ca(s) + 2H†(aq) → Ca²+ (aq) + H2(g) When 0.1437 g of Ca(s) is combined with enough acid solution to make 150.0 g of solution in a coffee-cup calorimeter, all of the calcium reacts, raising the temperature from 21.80 °C to 24.90 °C. Assume that the solution is mostly water, so that the heat capacity is 4.184 J · g¬1.°C-1.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 59E: In a coffee-cup calorimeter, 50.0 mL of 0.100 M AgNO3 and 50.0 mL of 0.100 M HCl are mixed to yield...
Related questions
Question
100%
Determine ΔrH for the reaction (at 298 KK).
THEN Determine ΔrU for the reaction (at 298 KK).
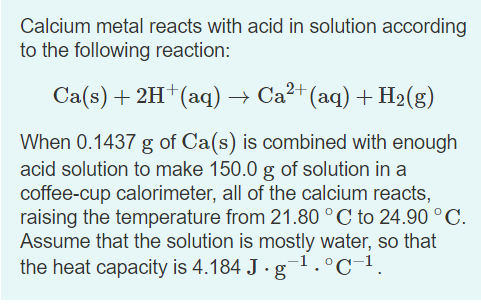
Transcribed Image Text:Calcium metal reacts with acid in solution according
to the following reaction:
Ca(s) + 2H†(aq) → Ca²+ (aq) + H2(g)
When 0.1437 g of Ca(s) is combined with enough
acid solution to make 150.0 g of solution in a
coffee-cup calorimeter, all of the calcium reacts,
raising the temperature from 21.80 °C to 24.90 °C.
Assume that the solution is mostly water, so that
the heat capacity is 4.184 J · g-1.°C-1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
