Calculate how many percent of each stereoisomer will be present in the product from the following reaction. Cat. A EIO,C CI (20 mol%) EIO2 EIOAC 'CI CI Yield = 78% dr =11:1 ee = 99% Ph .N. Cat. A O=
Calculate how many percent of each stereoisomer will be present in the product from the following reaction. Cat. A EIO,C CI (20 mol%) EIO2 EIOAC 'CI CI Yield = 78% dr =11:1 ee = 99% Ph .N. Cat. A O=
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 5CTQ
Related questions
Question
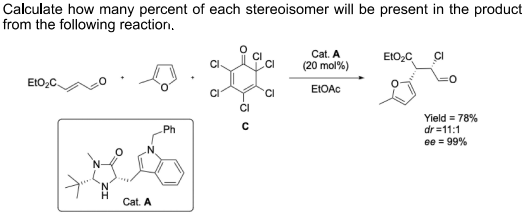
Transcribed Image Text:Calculate how many percent of each stereoisomer will be present in the product
from the following reaction.
Cat. A
EIO,C
CI
(20 mol%)
EIO2
EIOAC
'CI
CI
Yield = 78%
dr =11:1
ee = 99%
Ph
.N.
Cat. A
O=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning