Chapter5: Equilibrium, Activity And Solving Equations
Section: Chapter Questions
Problem 2P
Related questions
Question
Please answer the question below attatched is the mulitstep reaction.
Preparation of an Alcohol from Butyl Magnesium Bromide
- DATA
- Starting Materials
mass butylbromide _13.5942 g______________________________
mass Mg ____2.5967____g______________________________________
mass acetone __6.1123_g__________________________________
- Product
appearance and physical state __oily, clear and slightly yellow liquid,__________________________
b.p. obs. ______138 – 141 deg C.________________ method__distillation______________
b.p. pub. _ 141-142 °C_______________________ source __Chem spider_____________
- Calculate % yield: Based on amount of butylbromide used ____________
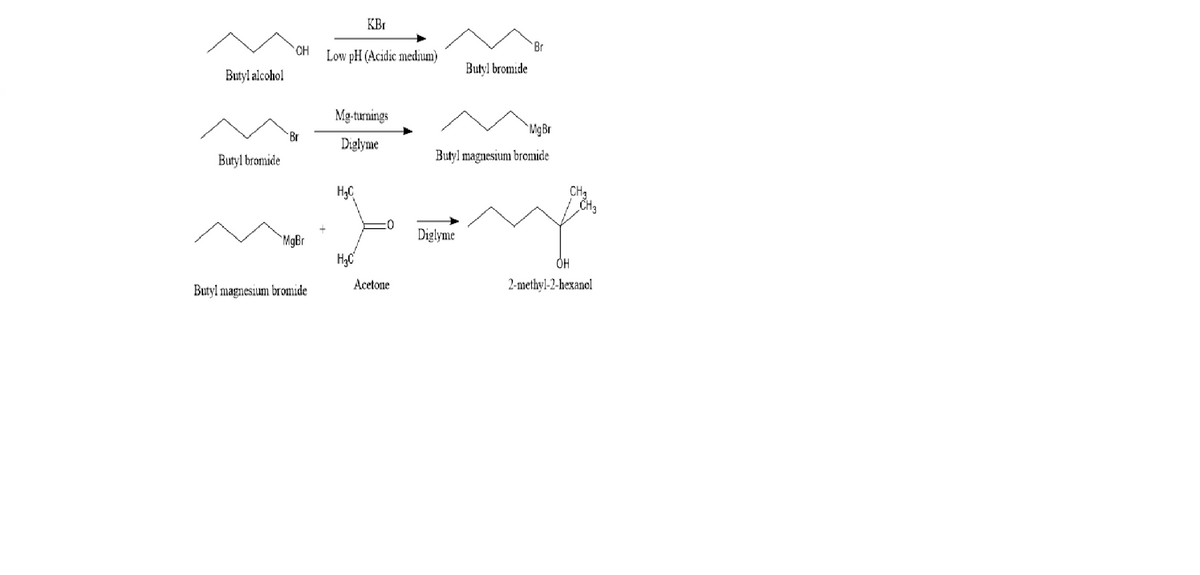
Transcribed Image Text:KBr
HO.
Br
Low pH (Acidic medium)
Butyl bromide
Butyl alcohol
Mg-turnings
MgBr
Br
Diglyme
Butyl bromide
Butyl magnesium bromide
HgC
CH3
CH3
"MgBr
Diglyme
Butyl magnesium bromide
Acetone
2-methyl-2-hexanol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you





