Calculate the activity coefficient, y, of Ni²+ when the ionic strength of the solution, u, is 0.085 M by linear interpolation Ionic Activity strength coefficient of the data in the table. (H, M) (7NP+) 0.001 0.870 0.005 0.749 YNi?+ = 0.01 0.675 0.05 0.485 0.1 0.405 Calculate the activity coefficient, 7, of Ni²+ when the ionic strength of the solution, u, is 0.085 M by using the extended Debye-Hückel equation at 25 °C, where the ion size is 600 pm. YN =.
Calculate the activity coefficient, y, of Ni²+ when the ionic strength of the solution, u, is 0.085 M by linear interpolation Ionic Activity strength coefficient of the data in the table. (H, M) (7NP+) 0.001 0.870 0.005 0.749 YNi?+ = 0.01 0.675 0.05 0.485 0.1 0.405 Calculate the activity coefficient, 7, of Ni²+ when the ionic strength of the solution, u, is 0.085 M by using the extended Debye-Hückel equation at 25 °C, where the ion size is 600 pm. YN =.
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.11QAP
Related questions
Question
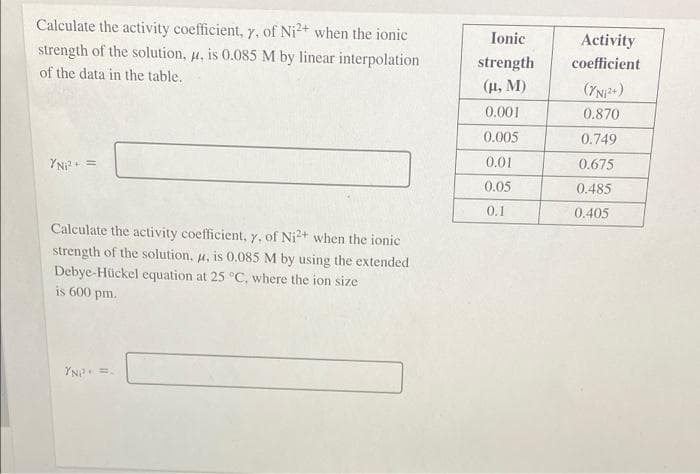
Transcribed Image Text:Calculate the activity coefficient, Y. of Ni2+ when the ionic
strength of the solution, u, is 0.085 M by linear interpolation
Ionic
Activity
strength
coefficient
of the data in the table.
(H, M)
0.001
0.870
0.005
0.749
YN?
0.01
0.675
0.05
0.485
0.1
0.405
Calculate the activity coefficient, y, of Ni2+ when the ionic
strength of the solution, u, is 0.085 M by using the extended
Debye-Hückel equation at 25 °C, where the ion size
is 600 pm.
YN =.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

