Calculate the average atomic mass of an element that has an isotope with a mass of 35.00 amu (75% abundance) and another isotope with a mass of 37.00 (25% abundance). What is this element?
Calculate the average atomic mass of an element that has an isotope with a mass of 35.00 amu (75% abundance) and another isotope with a mass of 37.00 (25% abundance). What is this element?
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 23E: Average atomic masses listed by JUPAC are based on a study of experimental results. Bromine has two...
Related questions
Question
100%
Calculate the average atomic mass of an element that has an isotope with a mass of 35.00 amu (75% abundance) and another isotope with a mass of 37.00 (25% abundance). What is this element? Make sure to prove this with your math calculations.
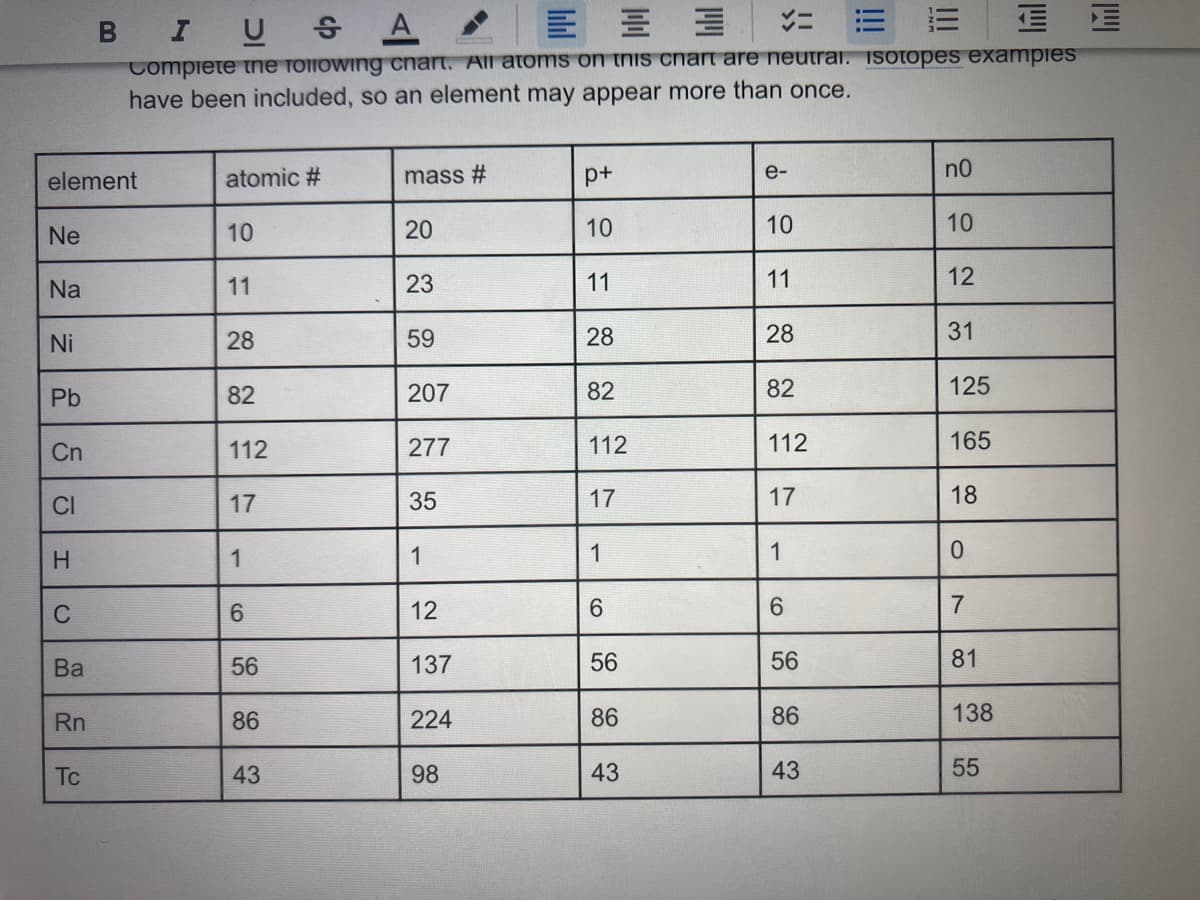
Transcribed Image Text:B IU
SA E I I
Compiete the following chart. All atoms on this chart are neutrai. Isotopes exampies
have been included, so an element may appear more than once.
element
atomic #
mass #
e-
n0
Ne
10
20
10
10
10
Na
11
23
11
11
12
Ni
28
59
28
28
31
Pb
82
207
82
82
125
Cn
112
277
112
112
165
CI
17
35
17
17
18
H.
1
1
1
1
C
12
6.
7
Ва
56
137
56
56
81
Rn
86
224
86
86
138
Tc
43
98
43
43
55
!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning