ganization of the Periodic Table Tamera v Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. Does any element with zS 92 match the description? If you checked yes, give the symbol of an element with zS 92 that matches. description An element in Period 2 and Group 8A. O Yes O No A nonmetal in Group 7A. O Yes O No An alkali metal with a lower atomic number than oxygen. O Yes O No A transition element in Period 6. O Yes O No Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility O 5:01 國山国
ganization of the Periodic Table Tamera v Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. Does any element with zS 92 match the description? If you checked yes, give the symbol of an element with zS 92 that matches. description An element in Period 2 and Group 8A. O Yes O No A nonmetal in Group 7A. O Yes O No An alkali metal with a lower atomic number than oxygen. O Yes O No A transition element in Period 6. O Yes O No Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility O 5:01 國山国
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 83SCQ
Related questions
Question
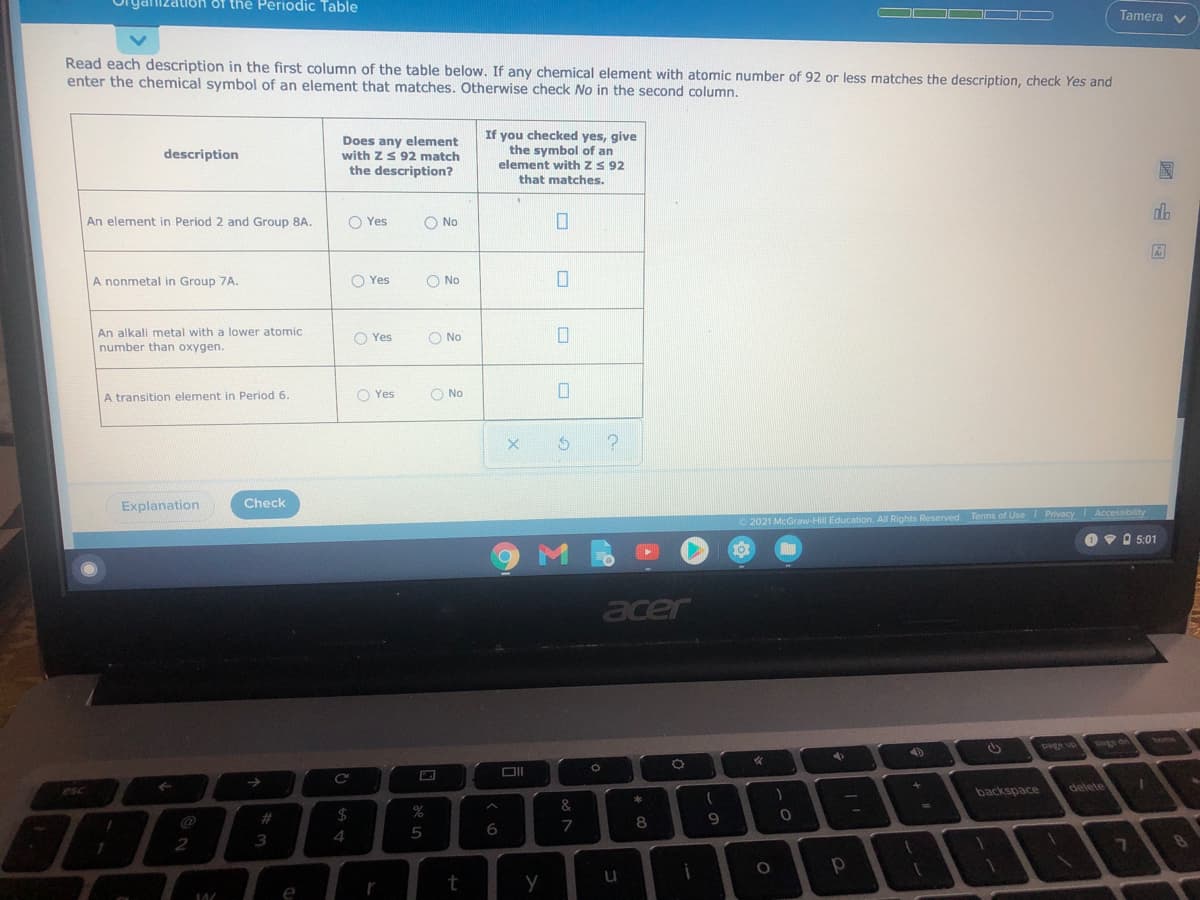
Transcribed Image Text:ganization of the Periodic Table
Tamera v
Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and
enter the chemical symbol of an element that matches. Otherwise check No in the second column.
Does any element
with ZS 92 match
the description?
If you checked yes, give
the symbol of an
element with zS 92
description
that matches.
An element in Period 2 and Group 8A.
O Yes
O No
A nonmetal in Group 7A.
O Yes
O No
An alkali metal with a lower atomic
number than oxygen.
O Yes
O No
A transition element in Period 6.
O Yes
O No
Explanation
Check
Privacy Accessibility
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use
O90 5:01
acer
o ed
home
n Bed
DII
backspace
delete
esc
&
9
5
6
7
3
t
e
国山回
Expert Solution
Step 1
The Atomic Number 92 is used for Uranium.
(1) An element in period 2 and group 8A -
Group 8A is of inert gases,or Noble gas , period second has Neon element with symbol of Ne.
(2) A non metal in group 7A -
Group 7A is known as halogens,it include fluorine, Chlorine, Bromine, Iodine etc ,these are nonmetallic.
(3) An alkali metal with lower atomic number than oxygen -
Oxygen has atomic number of 8, while it is present in period 2, so alkali metal is lithium, with symbol of Li.
(4) A transition element in period 6 -
In period 6 transition elements start with Lanthanum-57 to Mercury-80.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning