Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 16P: In the problem 15 above, what is vy , the y-component of the electron’s velocity, when it has...
Related questions
Question
Transcribed Image Text:course.html?courseld3172215518&OpenVellumHMAC=c19f920409449ebe43741778f1605e61#10001
Works Cited P..
G kenyon college - G..
G university of dayton.
G Xavier University of.
G howard university .
G kent state - Google..
Q Schedule Appoi
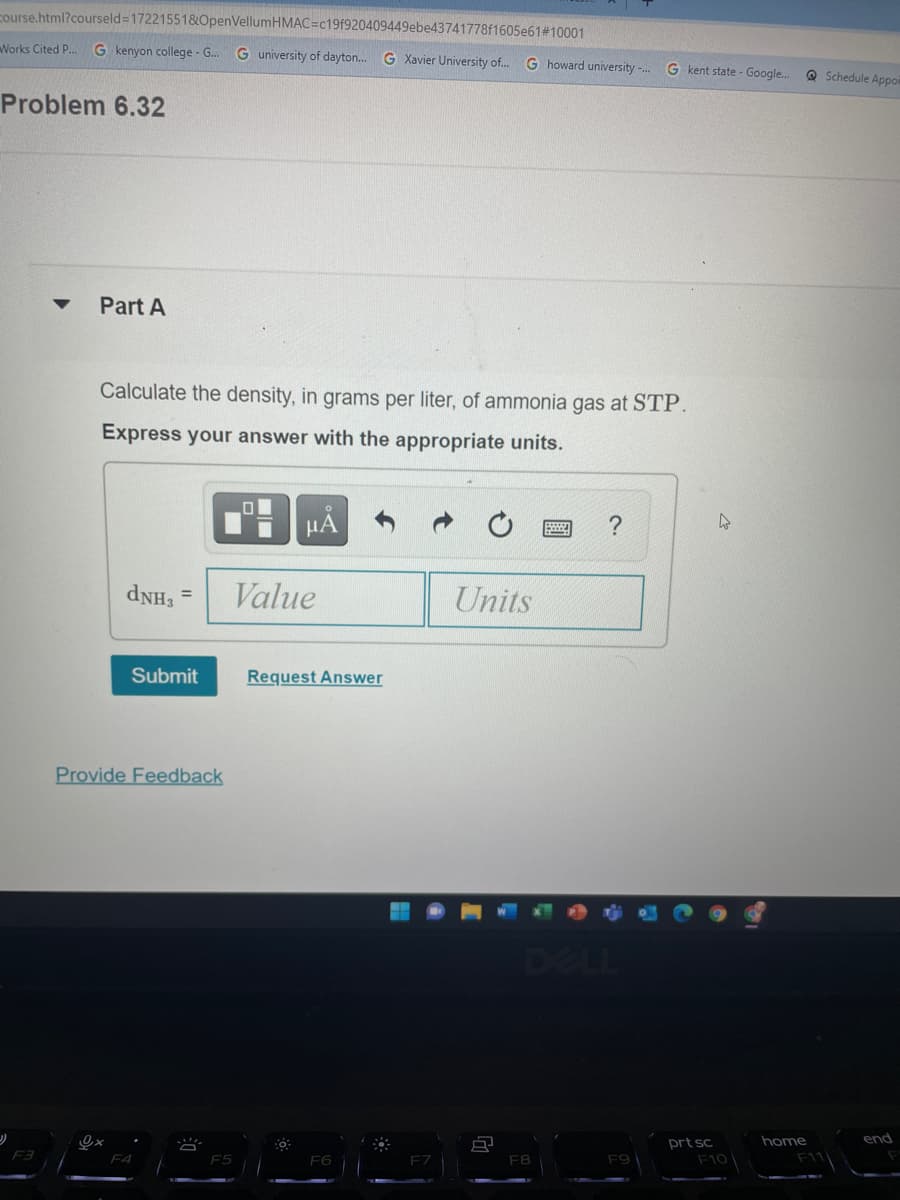
Problem 6.32
Part A
Calculate the density, in grams per liter, of ammonia gas at STP.
Express your answer with the appropriate units.
HẢ
Value
Units
Submit
Request Answer
Provide Feedback
DELL
prt sc
home
end
F3
F4
E6
F8
F9
F10
F11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning