Acid can dissociate in solution, which characterized by dissociation constant (Ka). Polyprotic acids can dissociate multiple times, which associated by multiple dissociation constant (Kal, Ka2, Ka3 etc.) CO2 dissolve in water forms carbonic acid H2CO3 CO2 (aq) = H2CO3 (aq) K-1.7x103 H2CO3 (aq) = HCO3 (aq) +H'(aq) Kal-2.5x104 HCO3 (aq) = CO3 (aq) + H Ka2 5.0x10-11 (aq) (a) For soda water, the CO2 concentration is 6g/L and the pH is 4. Calculate the equilibrium molar concentration of HCO; and CO32- (b) It is known that the CO2 concentration in air is 387ppm, and the CO2 Henry constant KH= 29.4 atm/mol. The second dissociation is negligible. Calculate the pH of the pure water after equilibrium in air 啟用
Acid can dissociate in solution, which characterized by dissociation constant (Ka). Polyprotic acids can dissociate multiple times, which associated by multiple dissociation constant (Kal, Ka2, Ka3 etc.) CO2 dissolve in water forms carbonic acid H2CO3 CO2 (aq) = H2CO3 (aq) K-1.7x103 H2CO3 (aq) = HCO3 (aq) +H'(aq) Kal-2.5x104 HCO3 (aq) = CO3 (aq) + H Ka2 5.0x10-11 (aq) (a) For soda water, the CO2 concentration is 6g/L and the pH is 4. Calculate the equilibrium molar concentration of HCO; and CO32- (b) It is known that the CO2 concentration in air is 387ppm, and the CO2 Henry constant KH= 29.4 atm/mol. The second dissociation is negligible. Calculate the pH of the pure water after equilibrium in air 啟用
Chapter3: Mechanisms
Section: Chapter Questions
Problem 31EQ: The reaction just described is reversible. Deprotonation of the conjugate acid of an organic base by...
Related questions
Question
5
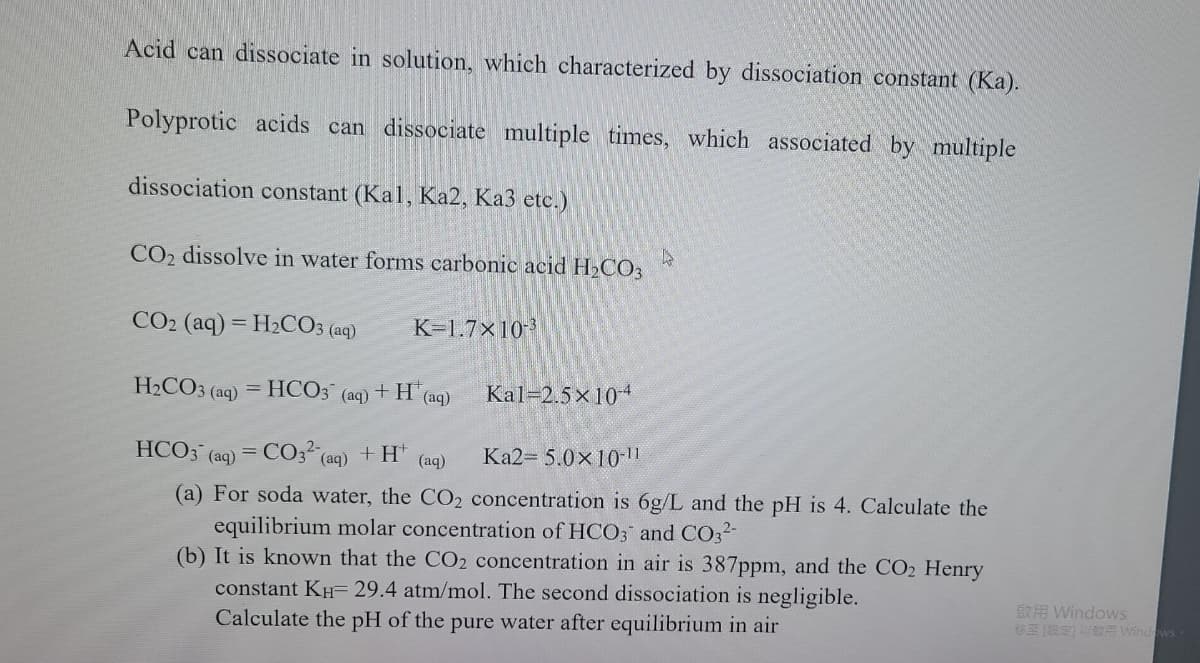
Transcribed Image Text:Acid can dissociate in solution, which characterized by dissociation constant (Ka).
Polyprotic acids can dissociate multiple times, which associated by multiple
dissociation constant (Kal, Ka2, Ka3 etc.)
CO2 dissolve in water forms carbonic acid H2CO3
CO2 (aq) = H2CO3 (aq)
K-1.7x10-
H2CO3 (aq)
= HCO3
+H (aq)
Kal-2.5x104
(aq)
HCO3 (aq) = CO3 (aq) + H" (ag)
Ka2- 5.0x10 ||
(a) For soda water, the CO2 concentration is 6g/L and the pH is 4. Calculate the
equilibrium molar concentration of HCO3 and CO32-
(b) It is known that the CO2 concentration in air is 387ppm, and the CO2 Henry
constant KH= 29.4 atm/mol. The second dissociation is negligible.
Calculate the pH of the pure water after equilibrium in air
ER Windows
BE R Windows-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning