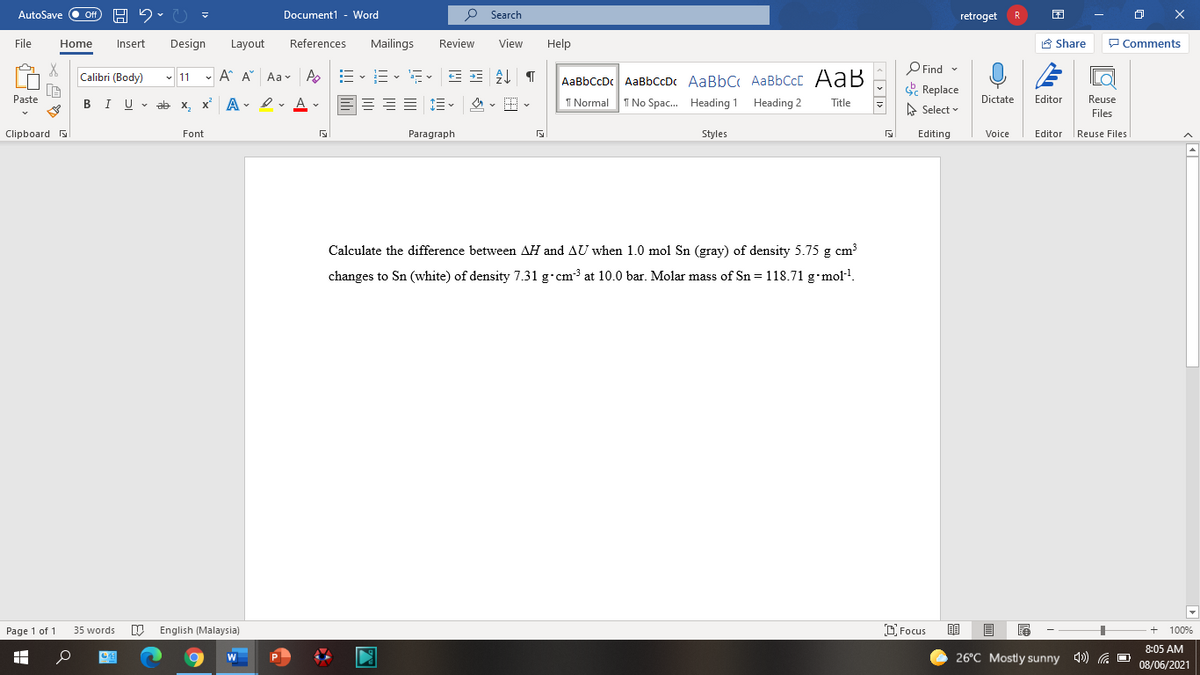
Calculate the difference between AH and AU when 1.0 mol Sn (gray) of density 5.75 g cm³ changes to Sn (white) of density 7.31 g-cm³ at 10.0 bar. Molar mass of Sn = 118.71 g-mol!.
Calculate the difference between AH and AU when 1.0 mol Sn (gray) of density 5.75 g cm³ changes to Sn (white) of density 7.31 g-cm³ at 10.0 bar. Molar mass of Sn = 118.71 g-mol!.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.77PAE
Related questions
Question
Transcribed Image Text:AutoSave
Document1 - Word
O Search
ff
retroget
R
File
Home
Insert
Design
Layout
References
Mailings
Review
View
Help
A Share
P Comments
O Find -
E Replace
Calibri (Body)
- 11 -
A A Aav A
AaBbCcDc AaBbCcDc AaBbC AaBbCcC AaB
Paste
I U v ab x, x A - e, v
A
1 Normal
I No Spac. Heading 1 Heading 2
Dictate
Editor
Reuse
Title
A Select
Files
Clipboard
Font
Paragraph
Styles
Editing
Editor Reuse Files
Voice
Calculate the difference between AH and AU when 1.0 mol Sn (gray) of density 5.75 g cm3
changes to Sn (white) of density 7.31 g•cm3 at 10.0 bar. Molar mass of Sn = 118.71 g•mol-!.
Page 1 of 1
W English (Malaysia)
D. Focus
35 words
100%
8:05 AM
W
26°C Mostly sunny 4) a O
08/06/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning