General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
Transcribed Image Text:+
ou add to the document will
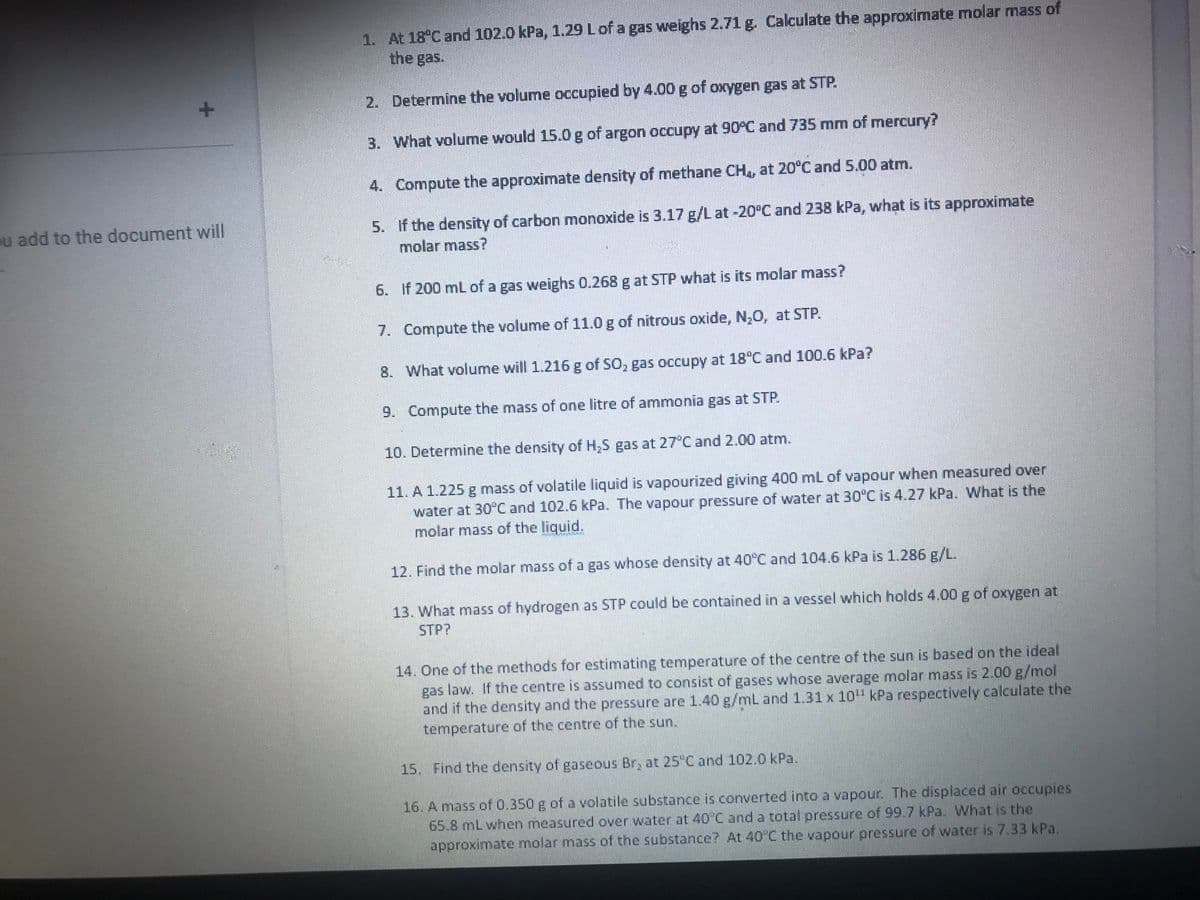
1. At 18°C and 102.0 kPa, 1.29 L of a gas weighs 2.71 g. Calculate the approximate molar mass of
the gas.
2. Determine the volume occupied by 4.00 g of oxygen gas at STP.
3.
What volume would 15.0 g of argon occupy at 90°C and 735 mm of mercury?
4. Compute the approximate density of methane CH4, at 20°C and 5.00 atm.
5. If the density of carbon monoxide is 3.17 g/L at -20°C and 238 kPa, what is its approximate
molar mass?
6.
If 200 mL of a gas weighs 0.268 g at STP what is its molar mass?
7.
Compute the volume of 11.0 g of nitrous oxide, N₂O, at STP.
8.
What volume will 1.216 g of SO, gas occupy at 18°C and 100.6 kPa?
9. Compute the mass of one litre of ammonia gas at STP.
10. Determine the density of H₂S gas at 27°C and 2.00 atm.
11. A 1.225 g mass of volatile liquid is vapourized giving 400 mL of vapour when measured over
water at 30°C and 102.6 kPa. The vapour pressure of water at 30°C is 4.27 kPa. What is the
molar mass of the liquid.
12. Find the molar mass of a gas whose density at 40°C and 104.6 kPa is 1.286 g/L.
13. What mass of hydrogen as STP could be contained in a vessel which holds 4.00 g of oxygen at
STP?
14. One of the methods for estimating temperature of the centre of the sun is based on the ideal
gas law. If the centre is assumed to consist of gases whose average molar mass is 2.00 g/mol
and if the density and the pressure are 1.40 g/mL and 1.31 x 10¹¹ kPa respectively calculate the
temperature of the centre of the sun.
15. Find the density of gaseous Br, at 25°C and 102.0 kPa.
16. A mass of 0.350 g of a volatile substance is converted into a vapour. The displaced air occupies
65.8 mL when measured over water at 40°C and a total pressure of 99.7 kPa. What is the
approximate molar mass of the substance? At 40°C the vapour pressure of water is 7.33 kPa.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
