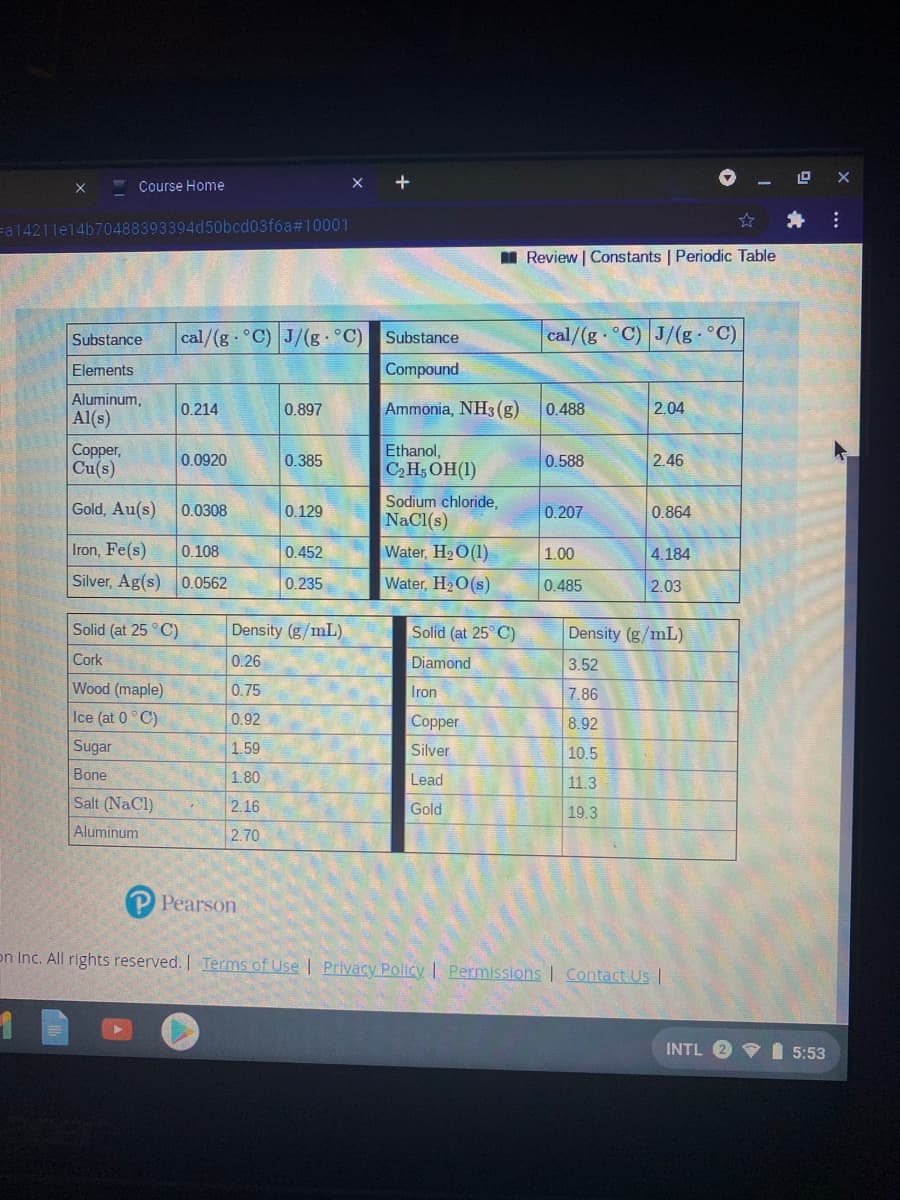
Calculate the energy needed to heat the cube of silver, with a volume of 10.0 cm, from 18 °C to 26 " C. Refer to the tables. Express the heat in calories to two significant figures.
Calculate the energy needed to heat the cube of silver, with a volume of 10.0 cm, from 18 °C to 26 " C. Refer to the tables. Express the heat in calories to two significant figures.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 16.CCP
Related questions
Question
Transcribed Image Text:Course Home
+
=a14211e14b70488393394d50bcd03f6a#10001
Review Constants | Periodic Table
Substance
cal/(g °C) J/(g °C)
cal/(g C) J/(g °C)
Substance
Elements
Compound
Aluminum,
0.214
0.897
Ammonia, NH3 (g)
0.488
2.04
Al(s)
Copper,
Cu(s)
Ethanol,
C,H; OH(1)
0.0920
0.385
0.588
2.46
Gold, Au(s)
Sodium chloride,
NaCl(s)
0.0308
0.129
0.207
0.864
Iron, Fe(s)
0.108
0.452
Water, H20(1)
1.00
4.184
Silver, Ag(s) 0.0562
0.235
Water, H2O(s)
0.485
2.03
Solid (at 25 ° C)
Density (g/mL).
Solid (at 25° C)
Density (g/mL)
Cork
0.26
Diamond
3.52
Wood (maple)
0.75
Iron
7.86
Ice (at 0°C)
0.92
Copper
8.92
Sugar
1.59
Silver
10.5
Bone
1.80
Lead
11.3
Salt (NaCl)
2.16
Gold
19.3
Aluminum
2.70
P Pearson
on Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions | Contact Us |
INTL 2 V 5:53
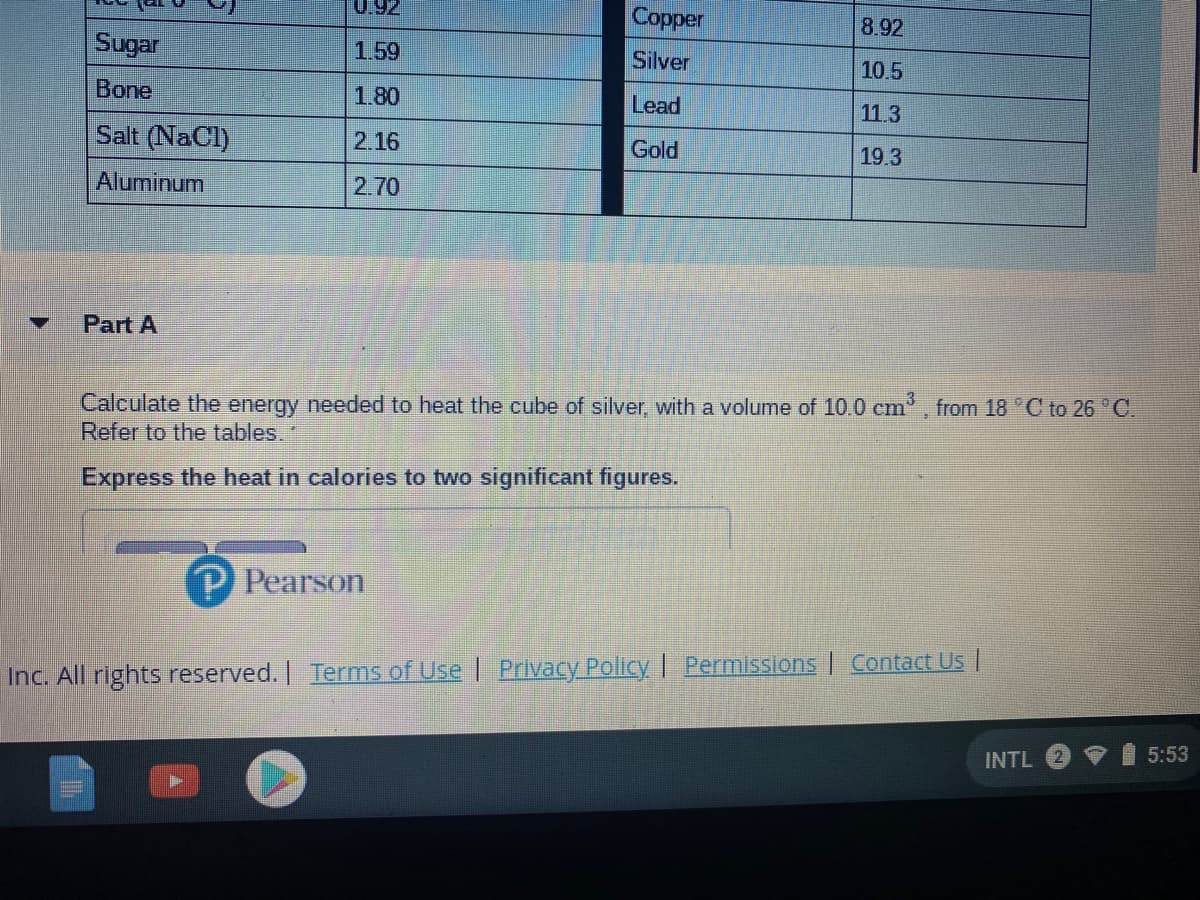
Transcribed Image Text:0.92
Copper
8.92
Sugar
1.59
Silver
10.5
Bone
1.80
Lead
11.3
Salt (NaCl)
2.16
Gold
19.3
Aluminum
2.70
Part A
Calculate the energy needed to heat the cube of silver, with a volume of 10.0 cm, from 18 C to 26 C.
Refer to the tables.
Express the heat in calories to two significant figures.
P Pearson
Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions | Contact Us |
INTL
5:53
Expert Solution
Step 1
Given : Volume of silver cube = 10.0 cm3 = 10.0 mL (since 1 cm3 = 1 mL)
Initial temperature of the silver cube = 18 oC
And final temperature of the silver cube = 26 oC
Using the given tables, we can see the the density of silver is 10.5 g/mL and specific heat capacity of silver is 0.0562 cal/g.oC.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning