Calculate the energy of the photon emitted for each of the observed lines (based on its wavelength) and enter the photon's energy in the appropriate box below the wavelength. Express your calculated energy values correctly rounded to three significant figures and using Webassign's exponential notation. Enter your observations and calculations about the spectral lines in order from left to right as seen in the pictures provided.
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Calculate the energy of the photon emitted for each of the observed lines (based on its wavelength) and enter the photon's energy in the appropriate box below the wavelength. Express your calculated energy values correctly rounded to three significant figures and using Webassign's exponential notation. Enter your observations and calculations about the spectral lines in order from left to right as seen in the pictures provided.
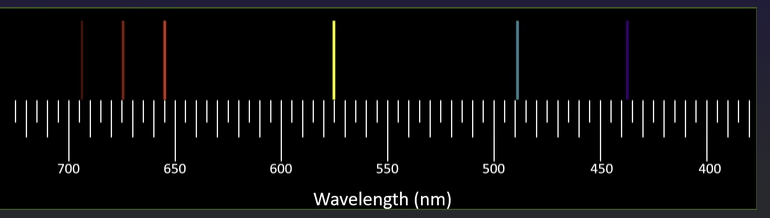
Interpretation - To calculate the energy of the photon emitted for each of the observed lines (based on its wavelength).
Relation between energy of a photon and wavelength is given by-
Step by step
Solved in 3 steps









