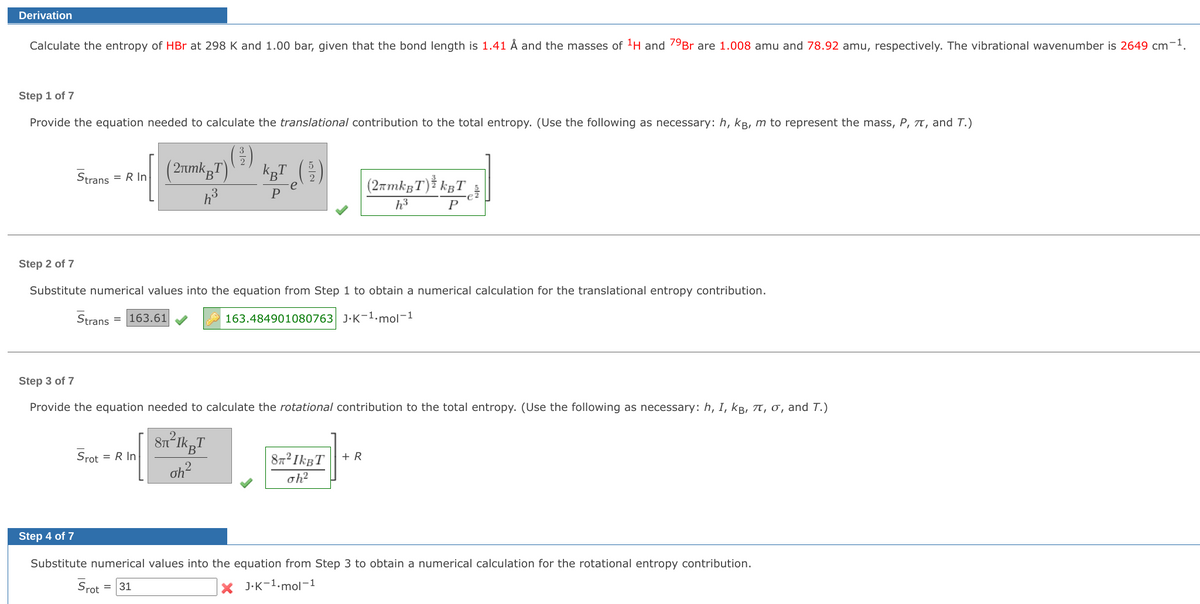
Calculate the entropy of HBr at 298 K and 1.00 bar, given that the bond length is 1.41 Å and the masses of ¹H and 79Br are 1.008 amu and 78.92 amu, respectively. The vibrational wavenumber is 2649 cm-1. Step 1 of 7 Provide the equation needed to calculate the translational contribution to the total entropy. (Use the following as necessary: h, kg, m to represent the mass, P, 7t, and T.) (2017)(2) +27 (2) (2μmkBT) Strans = R In (2æmkâT)¾ kgT_³ h³ P h³ P Step 2 of 7 Substitute numerical values into the equation from Step 1 to obtain a numerical calculation for the translational entropy contribution. Strans = 163.61✔ 163.484901080763 J-K-1-mol-1 Step 3 of 7 Provide the equation needed to calculate the rotational contribution to the total entropy. (Use the following as necessary: h, I, kB, π, σ, and T.) Srot = R In 8n²IkgT oh² +R 8² IkBT oh² ✓ Step 4 of 7 Substitute numerical values into the equation from Step 3 to obtain a numerical calculation for the rotational entropy contribution. Srot = 31 XJ-K-1-mol-1
Calculate the entropy of HBr at 298 K and 1.00 bar, given that the bond length is 1.41 Å and the masses of ¹H and 79Br are 1.008 amu and 78.92 amu, respectively. The vibrational wavenumber is 2649 cm-1. Step 1 of 7 Provide the equation needed to calculate the translational contribution to the total entropy. (Use the following as necessary: h, kg, m to represent the mass, P, 7t, and T.) (2017)(2) +27 (2) (2μmkBT) Strans = R In (2æmkâT)¾ kgT_³ h³ P h³ P Step 2 of 7 Substitute numerical values into the equation from Step 1 to obtain a numerical calculation for the translational entropy contribution. Strans = 163.61✔ 163.484901080763 J-K-1-mol-1 Step 3 of 7 Provide the equation needed to calculate the rotational contribution to the total entropy. (Use the following as necessary: h, I, kB, π, σ, and T.) Srot = R In 8n²IkgT oh² +R 8² IkBT oh² ✓ Step 4 of 7 Substitute numerical values into the equation from Step 3 to obtain a numerical calculation for the rotational entropy contribution. Srot = 31 XJ-K-1-mol-1
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter13: Spontaneous Processes And Thermodynamic Equilibrium
Section: Chapter Questions
Problem 60AP
Related questions
Question
Transcribed Image Text:Derivation
Calculate the entropy of HBr at 298 K and 1.00 bar, given that the bond length is 1.41 Å and the masses of ¹H and 79Br are 1.008 amu and 78.92 amu, respectively. The vibrational wavenumber is 2649 cm-¹.
Step 1 of 7
Provide the equation needed to calculate the translational contribution to the total entropy. (Use the following as necessary: h, kB, m to represent the mass, P, T, and T.)
(²³1)
(2πmkT)
h³
Strans = R In
kµT (²/2)
e
P
(2πmkBT) ³ kBT
h³
P
Step 2 of 7
Substitute numerical values into the equation from Step 1 to obtain a numerical calculation for the translational entropy contribution.
Strans = 163.61
163.484901080763 J.K-1.mol-1
Step 3 of 7
Provide the equation needed to calculate the rotational contribution to the total entropy. (Use the following as necessary: h, I, kB, 7, σ, and T.)
Srot
= R In
8n²lkpT
oh²
+ R
87² IkBT
oh²
Step 4 of 7
Substitute numerical values into the equation from Step 3 to obtain a numerical calculation for the rotational entropy contribution.
X J.K-¹.mol-1
Srot
= 31
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,