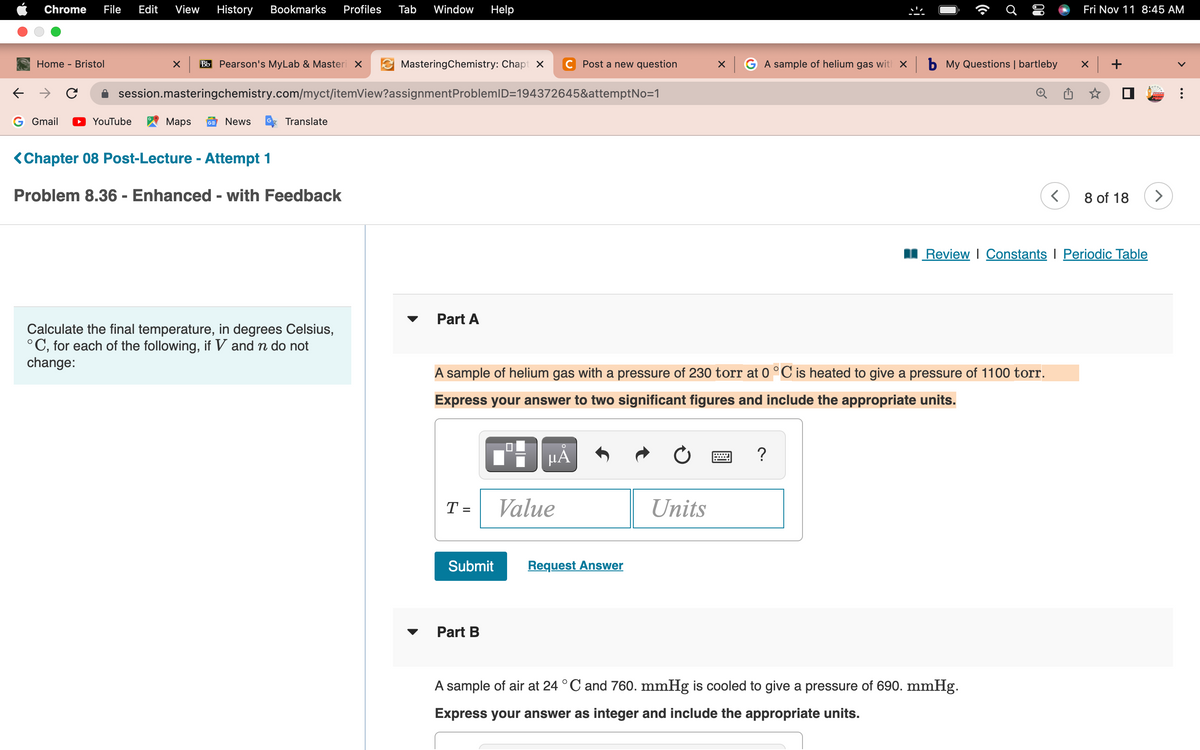
Calculate the final temperature, in degrees Celsius, °C, for each of the following, if V and n do not change: Part A A sample of helium gas with a pressure of 230 torr at 0 °C is heated to give a pressure of 1100 torr. Express your answer to two significant figures and include the appropriate units. μÅ T= Submit Part B Value Request Answer Units ? A sample of air at 24 °C and 760. mmHg is cooled to give a pressure of 690. mmHg. Express your answer as integer and include the appropriate units.
Calculate the final temperature, in degrees Celsius, °C, for each of the following, if V and n do not change: Part A A sample of helium gas with a pressure of 230 torr at 0 °C is heated to give a pressure of 1100 torr. Express your answer to two significant figures and include the appropriate units. μÅ T= Submit Part B Value Request Answer Units ? A sample of air at 24 °C and 760. mmHg is cooled to give a pressure of 690. mmHg. Express your answer as integer and include the appropriate units.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Please answer A and B question thank you
Transcribed Image Text:←
Chrome File Edit View History Bookmarks Profiles Tab Window Help
Home - Bristol
→ C
G Gmail
X Bb Pearson's MyLab & Masteri X
YouTube
session.masteringchemistry.com/myct/itemView?assignment ProblemID=194372645&attemptNo=1
Maps GE News
Translate
<Chapter 08 Post-Lecture - Attempt 1
Problem 8.36 - Enhanced - with Feedback
MasteringChemistry: Chapt X
Calculate the final temperature, in degrees Celsius,
°C, for each of the following, if V and n do not
change:
Part A
T =
Submit
Part B
Post a new question
µÅ
Value
A sample of helium gas with a pressure of 230 torr at 0 °C is heated to give a pressure of 1100 torr.
Express your answer to two significant figures and include the appropriate units.
Request Answer
Ć
Units
(co
?
♂
០១
x G A sample of helium gas with × b My Questions | bartleby x +
A sample of air at 24 °C and 760. mmHg is cooled to give a pressure of 690. mmHg.
Express your answer as integer and include the appropriate units.
Fri Nov 11 8:45 AM
<
Review | Constants I Periodic Table
8 of 18
>
<
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

