Calculate the molar solubility of iron) hydroxide (Fe(OH)) if the K is 6.012 10 M at room temperature, and provide the following information: al The balanced solubility equilibrium reaction (include the stokchiometric coefficient with the formula, exceat if the stoichiometric coefficient is 1 (s) = (aq) + (aq) bị The K expression (put lons in square brackets, indicates 'to the power of: d Solubility: (value, unit)
Calculate the molar solubility of iron) hydroxide (Fe(OH)) if the K is 6.012 10 M at room temperature, and provide the following information: al The balanced solubility equilibrium reaction (include the stokchiometric coefficient with the formula, exceat if the stoichiometric coefficient is 1 (s) = (aq) + (aq) bị The K expression (put lons in square brackets, indicates 'to the power of: d Solubility: (value, unit)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 70AP
Related questions
Question
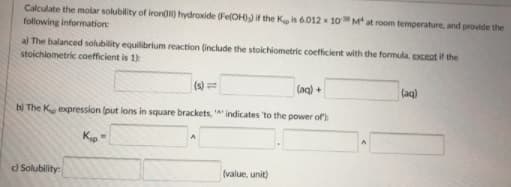
Transcribed Image Text:Calculate the molar solubility of iron) hydroxide (Fe(OH)) if the K is 6.012 10 M at room temperature, and provide the
following information:
al The balanced solubility equilibrium reaction (include the stokchiometric coefficient with the formula, exceat if the
stoichiometric coefficient is 1
(s) =
(aq) +
(aq)
bị The K expression (put lons in square brackets, indicates 'to the power of:
d Solubility:
(value, unit)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning