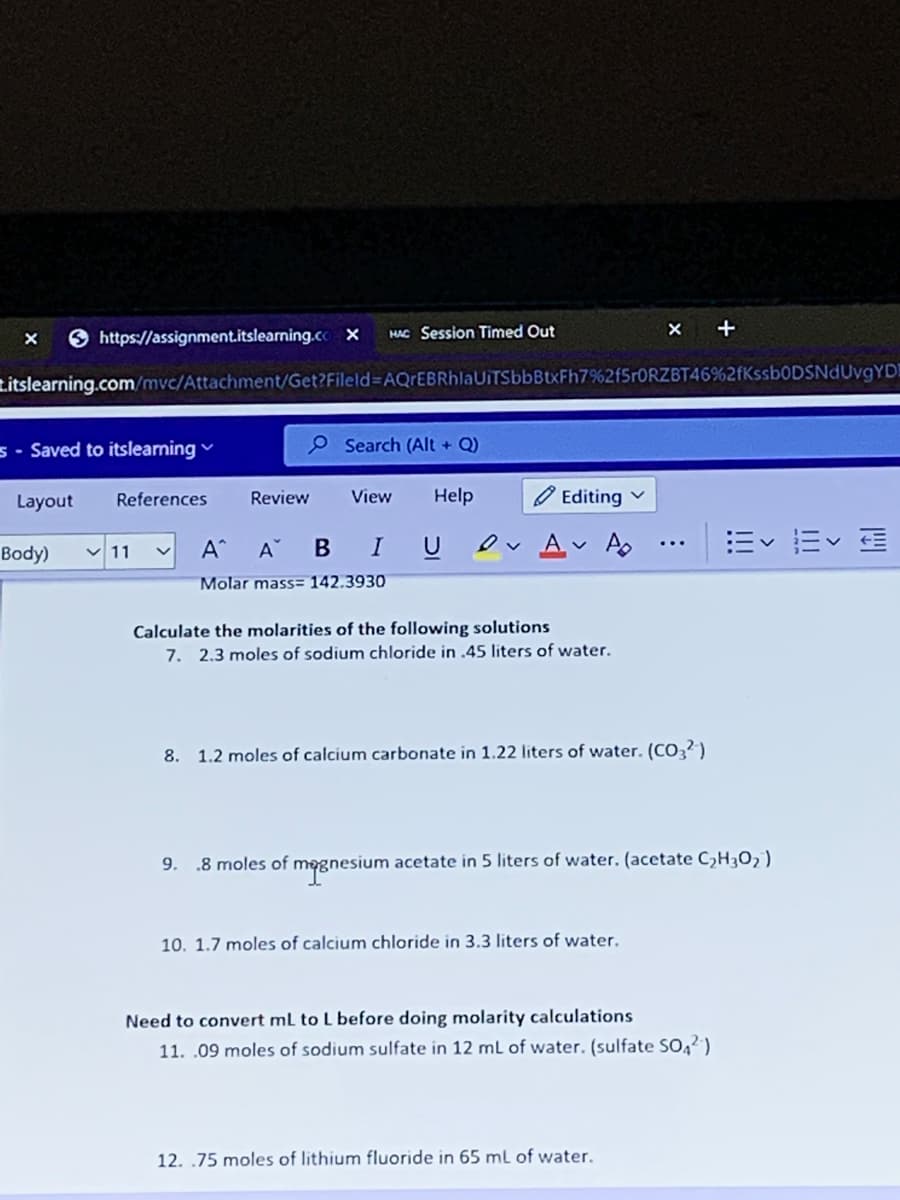
Calculate the molarities of the following solutions 7. 2.3 moles of sodium chloride in .45 liters of water. 8. 1.2 moles of calcium carbonate in 1.22 liters of water. (CO3?) .8 moles of megnesium acetate in 5 liters of water. (acetate C,H302)
Calculate the molarities of the following solutions 7. 2.3 moles of sodium chloride in .45 liters of water. 8. 1.2 moles of calcium carbonate in 1.22 liters of water. (CO3?) .8 moles of megnesium acetate in 5 liters of water. (acetate C,H302)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.108PAE: 3.108 As chip speeds increase, the width of the interconnects described in Problem 3.107 must be...
Related questions
Question
100%
need help how to solve 7, 8, 9
Transcribed Image Text:+
O https://assignment.itslearning.co x
HAC Session Timed Out
zitslearning.com/mvc/Attachment/Get?Fileld AQrEBRhlaUITSbbBtxFh7%2f5r0RZBT46%2fKssb0DSNdUvgYD!
s - Saved to itslearning
9 Search (Alt + Q)
Layout
References
Review
View
Help
Editing
U Ov Av Ao ..
Ev E E
Body)
v 11
A^
A
Molar mass= 142.3930
Calculate the molarities of the following solutions
7. 2.3 moles of sodium chloride in .45 liters of water.
8. 1.2 moles of calcium carbonate in 1.22 liters of water. (CO3?)
9. .8 moles of megnesium acetate in 5 liters of water. (acetate C,H30, )
10. 1.7 moles of calcium chloride in 3.3 liters of water.
Need to convert mL to L before doing molarity calculations
11. .09 moles of sodium sulfate in 12 mL of water. (sulfate SO,2)
12. .75 moles of lithium fluoride in 65 ml of water.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning