A)What is the H+H+ concentration for an aqueous solution with pOHpOH = 3.54 at 25 ∘C∘C? Express your answer to two significant figures and inclu
A)What is the H+H+ concentration for an aqueous solution with pOHpOH = 3.54 at 25 ∘C∘C? Express your answer to two significant figures and inclu
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 10PE
Related questions
Question
A)What is the H+H+ concentration for an aqueous solution with pOHpOH = 3.54 at 25 ∘C∘C?
Express your answer to two significant figures and include the appropriate units.
b)At a certain temperature, the pHpH of a neutral solution is 7.49. What is the value of KwKw at that temperature?
Express your answer numerically to two significant figures.
c) answer the attached image
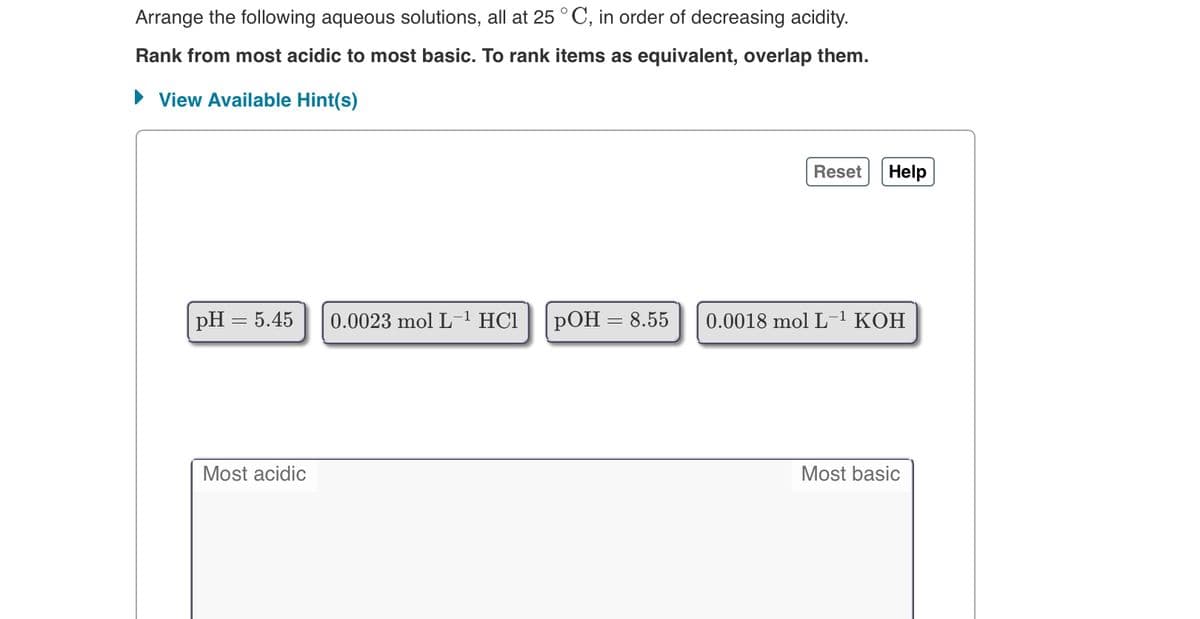
Transcribed Image Text:Arrange the following aqueous solutions, all at 25 °C, in order of decreasing acidity.
Rank from most acidic to most basic. To rank items as equivalent, overlap them.
• View Available Hint(s)
Reset
Help
pH = 5.45
0.0023 mol L
HC1
РОН
8.55
0.0018 mol L-1 KOH
Most acidic
Most basic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning