calculate the molarity of the oxalic acid standard solution.
Chapter12: Water Requirements For Aquaculture
Section: Chapter Questions
Problem 27SA
Related questions
Question
Using this data, calculate the molarity of the oxalic acid standard solution.
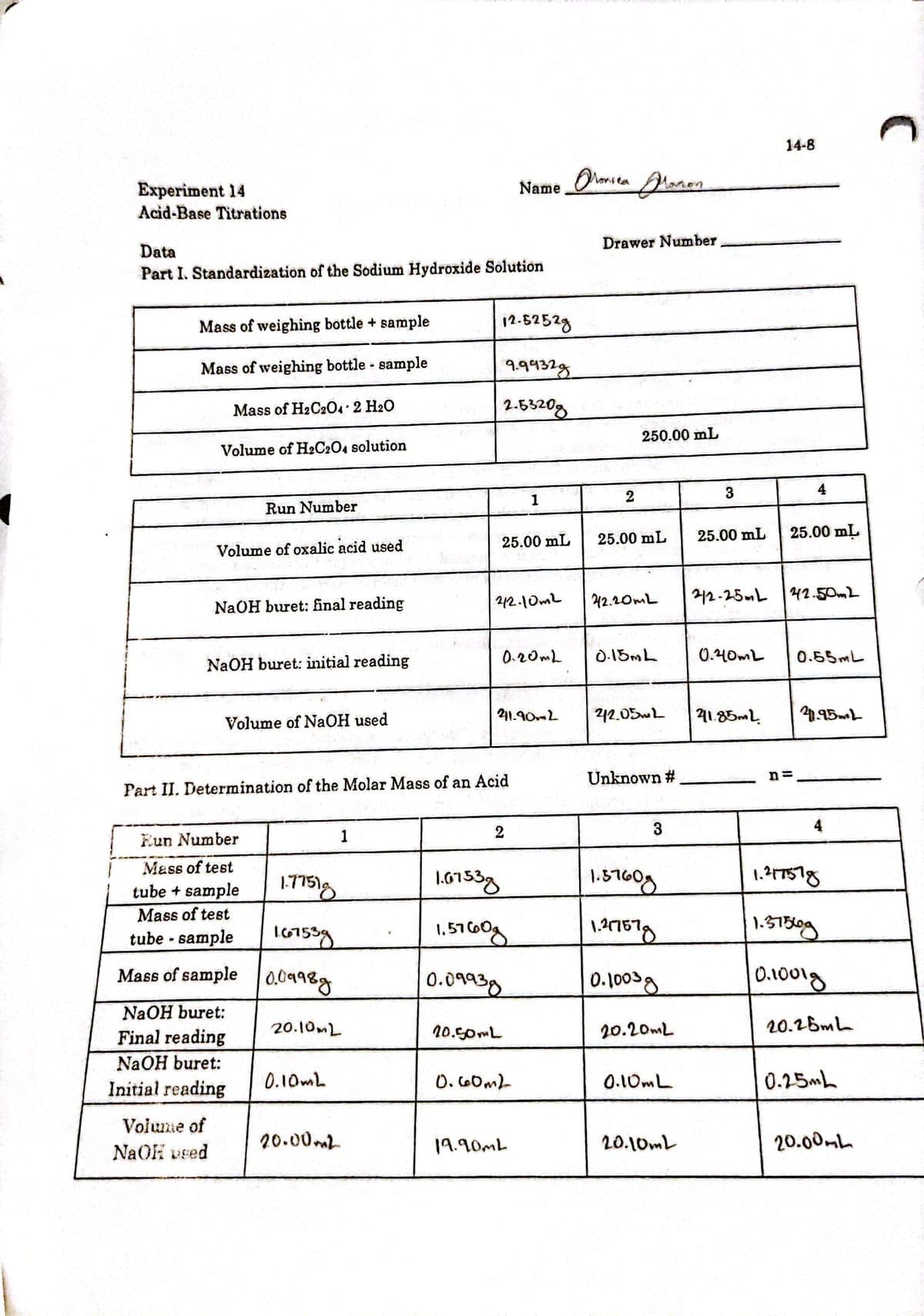
Transcribed Image Text:14-8
Experiment 14
Name Olonsea
Acid-Base Titrations
Drawer Number
Data
Part I. Standardization of the Sodium Hydroxide Solution
Mass of weighing bottle + sample
12.5252g
Mass of weighing bottle - sample
9432g
Mass of H2C20 2 H20
2.5320g
250.00 mL
Volume of H2C204 solution
2
3
4
1
Run Number
25.00 mL
25.00 mL
25.00 mL
25.00 mL
Volume of oxalic acid used
42.20mL
212-25mL 42-50m2
NaOH buret: final reading
212.10mL
0.20mL
D.15ML
0.40mL
0.55ML
NaOH buret: initial reading
211.90L
242.05wL
41.85mL
Volume of NaOH used
Unknown #
n=
Part II. Determination of the Molar Mass of an Acid
2
3
4
Eun Number
1
Mass of test
1.417578
tube + sample
Mass of test
tube - sample
1,5160g
1.S13og
Mass of sample
0,0998g
0.09939
0.1003g
0.1001g
NaOH buret:
20.10ML
20.50ML
20.20mL
20.26ML
Final reading
NaOH buret:
Initial reading
0.10mL
O. 60ml-
0.10ML
0.25mh
Volume of
20.00mL
19.90ML
20.10mL
20.00mL
NaOH vsed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage



Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage
