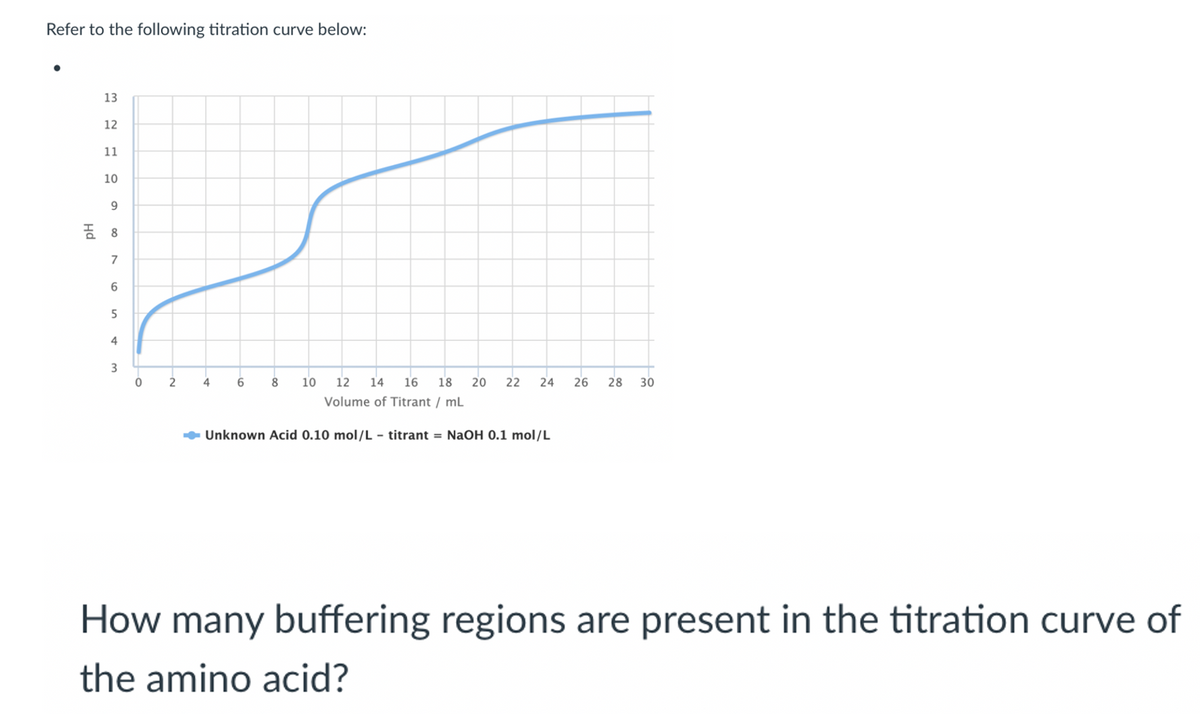
Refer to the following titration curve below: 13 12 11 10 7 5 4 3 2 4 6 10 12 14 16 18 20 22 24 26 28 30 Volume of Titrant / ml - Unknown Acid 0.10 mol/L - titrant = NaOH 0.1 mol/L How many buffering regions are present in the titration curve of the amino acid?
Q: How much of a 10x buffer should be added to 900ml of water to make a 1x solution?
A: In this question, we are given with- 1. Concentration of stock solution= 10X 2. Required…
Q: For the following, calculate pka, and pka, of 1 Glycine, determine the 2 buffering regions, and…
A: Glycine is an amino acid with H-atom as the side chain. The isoelectric point of a amino acid (pI)…
Q: A 100-mL buffer solution with pH of 4.80 is prepared as a stock solution. Using this stock buffer…
A: Hi! Thank you for the question. We are authorized to answer two subparts at a time, since you have…
Q: hat mass of sodium glycolate (NaC2H3O3) should be added to 400.0 mL of 1.00 M glycolic acid to…
A: We have given [ C2 H4 O3 = 1.00 , Volume= 4.00mL, pH=4.0
Q: Given a stock protein solution with a concentration of 3 mg/ml, determine the protein concentration…
A: Stock solutions are used in many laboratory procedures, in order to save space for keeping larger…
Q: A 7 D
A: A titration curve means it has a molecule that is titrated either with an acid or base. Acid and…
Q: Based on the pka values of the amino acids, is there any amino acid that could serve as a buffer at…
A: Amino acids are chemical molecules with amino and carboxyl functional groups as well as a side chain…
Q: 439 mL of 0.270 M hydrochloric acid is exactly neutralized with 0.920 M potassium hydroxide…
A: Given Values: Volume of HCl = 439 ml The concentration of HCl = 0.270 M Concentration of NaOH =…
Q: If the pH of a voledronic acid solution is 5.8, and the voledronate concentration is 9 mM, what is…
A: Henderson Hasselbalch equation can be used to determine the concentration of a weak acid in an…
Q: ou have given two proteins with a pI of 4.5 and pI of 7.7? Using an anion-exchange column, how can…
A: Ion-exchange chromatography: The method involves the separation of ions and polar molecules based…
Q: Using chemical equations, explain how bicarbonate ion and carbonic acid function as a buffer pair.
A:
Q: Write the chemical equation for the carbonic acid–bicarbonate buffer system?
A: When a strong acid is introduced into a body, buffers take care of it. Buffers mainly act as…
Q: What is the relationship betweenpKa and the useful range of a buffer?
A: The strength of an acid in a solution can be expressed in terms of the acid dissociation constant…
Q: How is Ka defined? Write the equation for Ka for the generalized acid HA.
A: Ka is considered as the equilibrium constant.
Q: What is the final concentration if 75 mL of a 3.5 M glucose solution is diluted to a volume of 450…
A: Dilution is the process of reducing the concentration of a given solute in its solution. It can be…
Q: amino glycine pKa is 2.4, 9.8 Calculate the most effective buffering range. if there is two pka,…
A: The amino acid is the smallest monomer of the protein, which acts as a buffer system.
Q: Calculate pH of buffer prepared by mixing; 10ml of 0.10M of CH3COOH and 20ml of 0.10M of CH3COONa.
A: A buffer is defined as the mixture of a weak acid and its conjugate base or weak base and its…
Q: Calculate the pH of a mixture of 0.25 M acetic acid and 0.20 M sodium acetate. The pKa of acetic…
A: The Hasselbalch-Henderson equation is used to estimate the pH of a buffer solution. The numerical…
Q: Which lipid sample is soluble or miscible in water? Explain.
A: Lipids are macro molecules that consists of monomers of fattyacids. Fattyacids are esterified to…
Q: What is the amount of dextrose in mg/kg/min provided by the following TPN for a 60 kg patient? Round…
A: Drug calculation is a important aspect in calculating the dose based on the BMI ,BSA ,age and body…
Q: determine the pKa of the acid and ph of the buffer sol'n of a buffer mixed with 0.1 M tris and 0.5 M…
A: Buffer solutions often are weak acid/strong acid and conjugate base. buffers resist the change…
Q: Would the carbonatelcarbonic acid conjugate base/weak acid system function as a good buffer at the…
A: A buffer is an aqueous solution that is formed of the mixture of weak acid and its conjugate base or…
Q: What is the functional difference between TAE and TBE buffer?
A: A buffer is a solution capable of withstanding pH changes even when a base or acid solution is…
Q: Define the following: - pH - Buffer - pKa
A: An acid-base reaction takes place between an acid and a base and is used to determine pH. An acid is…
Q: Calculate the pH of a dilute solution that contains a molar ratio of potassium acetate to acetic…
A: The Henderson-Hasselbalch equation depicts the measurement of acidity as pH in biochemical…
Q: A mixture of Alanine (pl 6.02), Glutamic Acid (pl 3.22), Glycine (pl 5.79), Lysine (pl 9.74) and…
A: Ion exchange chromatography is used to separate molecules based on their net surface charge.
Q: The greatest buffering capacity at physiological pH would be provided by a protein rich in which of…
A: Buffer capacity is a unitless number specified as the moles of acid or base used to change the pH of…
Q: The skeletal structures of the two amino acids, glycine and lysine, are given below along with the…
A: Amino acids are organic compounds that contain amine (-NH2) and carboxyl (-COOH) functional groups,…
Q: 12- 10- 8- 6- 4- 2- 0- 20 40 60 80 Volume of NaOH (mL) Hd
A: The titration curve is the graphical representation of pH. The pH is greater than 7 for weak acid…
Q: Describe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NAOH.…
A: Glycine-Sodium hydroxide buffer is a common buffer which has good buffering capacity. This buffer…
Q: Is lysine a suitable buffer at acidic, neutral, or basic pH range, and why?
A: The amino acid consists of both amino and carboxylic groups. It can act as a buffer because it can…
Q: Which of the following combinations would be the best choice to buffer the pH of a solution at…
A: Ans- NaH2PO4 and Na2HPO4 would be the best choice to buffer the pH of a solution because it has pka…
Q: The acid -base titration curve shown most likely resulted from 12- 10- 8- 6- 2- 01 60 20 Volume of…
A: An arrhenius acid donates a proton (H+), so a polyprotic acid donates protons.. However, a…
Q: Write the equations for reaction of this buffer with a small amount of HNO3 and with a small amount…
A: Nitric acid is a strong acid and sodium hydroxide is a strong base, both are considered as strong…
Q: Is lysine a suitable buffer at acidic, neutral, or basic pH range and why? What are the functions of…
A: Amino acids are the polymers that synthesize the proteins by joining together with the covalent…
Q: Given a stock protein solution with a concentration of 6 mg/ml, determine the protein concentration…
A: Given Values: Concentration of the protein stock solution = 6 mg/ml Volume of the stock protein…
Q: Given the equation of the line, y=150x-8.1221 (cells per mL is in 10^8), what is the CFU per mL of…
A: Spectrophotometry enables us to calculate the concentration of a species in a solution by measuring…
Q: Describe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NaOH.…
A: Molar concentration is a measure of the concentration of a chemical species, in particular of a…
Q: Table 2. Volume of BSA, protein content, and absorbance readings of reference solutions…
A: Standard curves are graphs of absorbance v/s concentration, It is used to find out the solute…
Q: What is the function of the buffer protein in the human body?
A: There are 3 types of buffer systems in our body. They are protein buffer system, phosphate buffer…
Q: C. Results Match each beaker with the set-up description in the Table 1 below based on the expected…
A: Acid is the substance that donates its proton, and an example of the acid is hydrochloric acid. The…
Q: A 1.0-L 0.010 M buffer with pH 6.50 is given as an assignment to a group of students. Which is the…
A: A weak acid is used to prevent any change in the pH of the solution, regardless of the solute. On…
Q: What is the correlation in hydration of proteins between the average weight and the ph? see the…
A: Myofibrillar protein : These are the main protein constituents of skeletal muscle tissue and…
Q: alanine that would exist at the pH indicated below.
A:
Q: Calculate the followings: The pH of the final solution after adding 300 ml of 40 mM NaOH to 600 ml…
A: pH is the scale that is used to specify the acidity or basicity of an aqueous solution. Acidic…
Q: If 4 mL of 1 M NaOH is added to 100 mL buffer, would it still be a usable buffer according to the…
A: A buffer can be destroyed by adding either a strong acid or a strong base in a large amount. The…
Q: What mass of sodium glycolate (NaC2H3O3) should be added to 400.0 mL of 1.00 M glycolic acid to…
A: We are given, [C2 H4 O3] = 1.00 M, Volume= 400.0 mL, pH = 4.00
Q: Which of the following match buffer solution: A- Consists of a mixture of a weak acid and its…
A: Introduction: The solution is a mixture of two compounds in which one dissolves the other. The…
Q: If the blood pH decreases in an acidosis from 7.42 to 7.15, what is the change in [H+] in nmol.l-1?
A: The homeostatic pH of the blood is maintained by several buffers that work in concert. The major…
Step by step
Solved in 2 steps with 1 images

- Given the following information, calculate the total activity in the undiluted protein sample. Activity of 1 ml of diluted sample = 0.5 Total volume of sample = 5 ml Dilution factor = 10 25 50.5 250 2.5Consider the following pH titration curve of a diprotic acid. What is the approximate values for pka 1 and pka 2? the curve is attached below.1.2. Using DEAE-cellulose as ion exchange resin, indicate the starting and ending pH for the narrowest experimental pH range used to separate an amino acid mixture consisting of Cys, His and Leu Starting pH: _____ Ending pH: _____
- Using this data, calculate the molarity of the oxalic acid standard solution.In biochemistry, what is definition of buffer , which amino acid can act as buffer? what is buffer pH range?The greatest buffering capacity at physiological pH would be provided by a protein rich in which of the following amino acids? Choose one from among the possible answers and explain. Serine Cysteine Alanine Histidine
- Table 2. Volume of BSA, protein content, and absorbance readings of reference solutions Solution Volume of BSA standard solution (μL) Protein content(μg/mL) Absorbance value At 595 nm 1 0 0 0 2 10 1 0.022 3 30 3 0.065 4 50 5 0.106 5 70 7 0.178 6 100 10 0.299 7 120 12 0.380 Make a graph by plotting the absorbance values versus the BSA protein content (in μg) for theseven reference solutions. When constructing the graph, be…Three buffers are made by combining a 1M solution of acetic acid and a 1M solution of sodium acetate in the ratios shown in the table below. Which of these statements is true regarding the prepared buffers? (Ka= 1.7x10-5) pH of buffer 1 < pH of buffer 2 < pH of buffer 3 pH of buffer 1 = pH of buffer 2 = pH of buffer 3 pH of buffer 1 > pH of buffer 2 > pH of buffer 3 pH of buffer 1 = pH of buffer 2 > pH of buffer 3 pH of buffer 1 > pH of buffer 2 = pH of buffer 3Using the equation for the best-fit straight line through your data, the average absorbance of your unknown samples U1 and U2, and any dilution factors (DON’T LEAVE OUT THE DILUTION FACTOR), calculate the concentration of protein in the original unknown protein sample. y = 1.6849x + 0.0414R² = 0.9904
- Is lysine a suitable buffer at acidic, neutral, or basic pH range, and why?In an experiment, what happens to the calculated molar mass after waiting an hour between thawing the frozen cyclohexane and adding the unknown compound, during which time some of the cyclohexane evaporated. Will the molar mass be too large, too small, or unaffected? Explain.|Explain in general why the two phenomena of salting in and salting out increase the solubility or deposition of proteins and compare the strength of different salts. (Hoffmister series)

