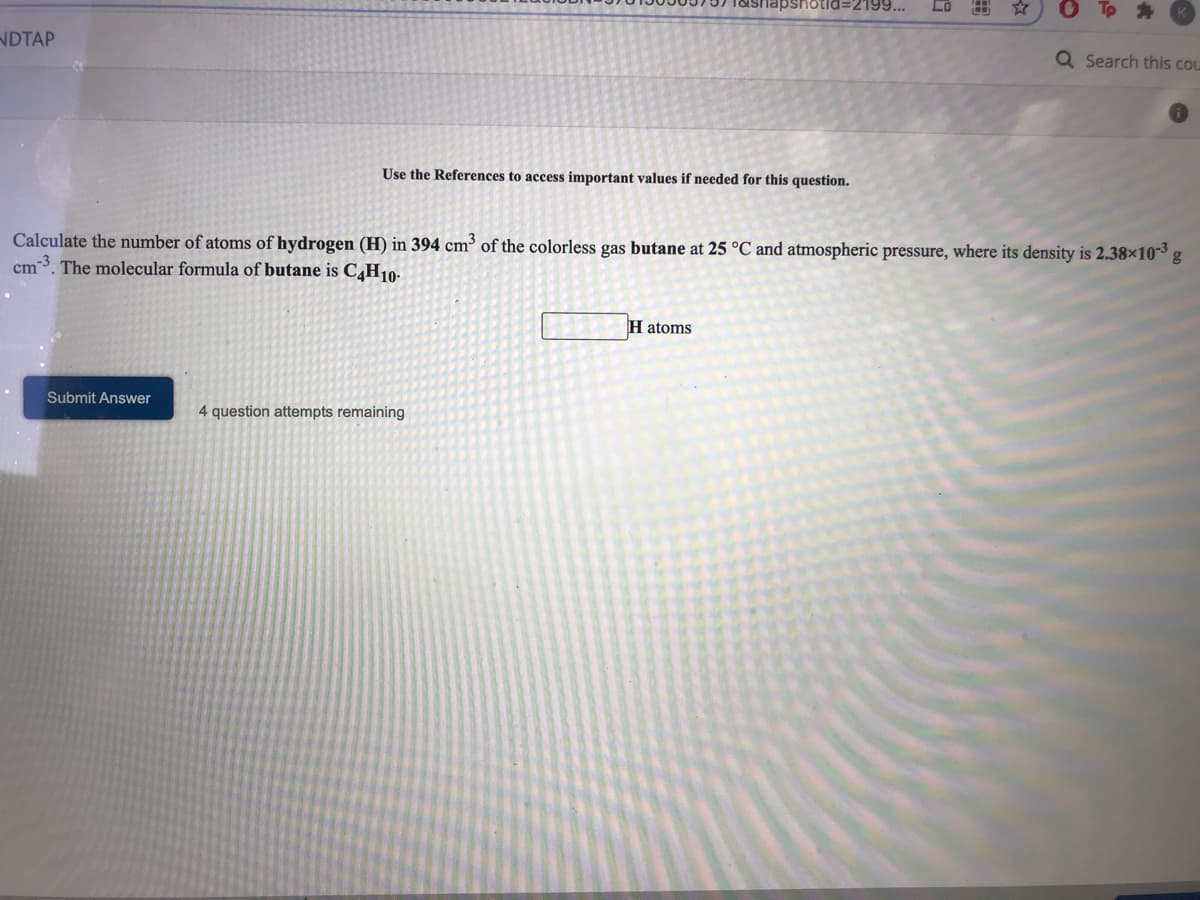
Calculate the number of atoms of hydrogen (H) in 394 cm³ of the colorless gas butane at 25 °C and atmospheric pressure, where its density is 2.38x103 cm 3. The molecular formula of butane is C,H10- H atoms Submit Answer 4 question attempts remaining
Calculate the number of atoms of hydrogen (H) in 394 cm³ of the colorless gas butane at 25 °C and atmospheric pressure, where its density is 2.38x103 cm 3. The molecular formula of butane is C,H10- H atoms Submit Answer 4 question attempts remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 25QAP: Cyclopropane mixed in the proper ratio with oxygen can be used as an anesthetic. At 755 mm Hg and...
Related questions
Question
Transcribed Image Text:pshotid=2199...
NDTAP
Q Search this cou
Use the References to access important values if needed for this question.
Calculate the number of atoms of hydrogen (H) in 394 cm³ of the colorless gas butane at 25 °C and atmospheric pressure, where its density is 2.38×10³ g
cm-3. The molecular formula of butane is C4H10-
H atoms
Submit Answer
4 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
