3.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 46. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 6.70 g water 4.11 g Use this information to find the molecular formula of X. do 00 Check Explanation Terms of Use Privacy O 2019 McGraw-Hill Education All Rights Reserved 11:52 PM 12/5/2019 99+ O Type here to search po end home ins prt sc delete 12 f9 fa f6 f5 13 * f2 num e backspace lock esc & 8 23 4 2 1 { O P home LO %24
3.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 46. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 6.70 g water 4.11 g Use this information to find the molecular formula of X. do 00 Check Explanation Terms of Use Privacy O 2019 McGraw-Hill Education All Rights Reserved 11:52 PM 12/5/2019 99+ O Type here to search po end home ins prt sc delete 12 f9 fa f6 f5 13 * f2 num e backspace lock esc & 8 23 4 2 1 { O P home LO %24
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 50A
Related questions
Question
100%
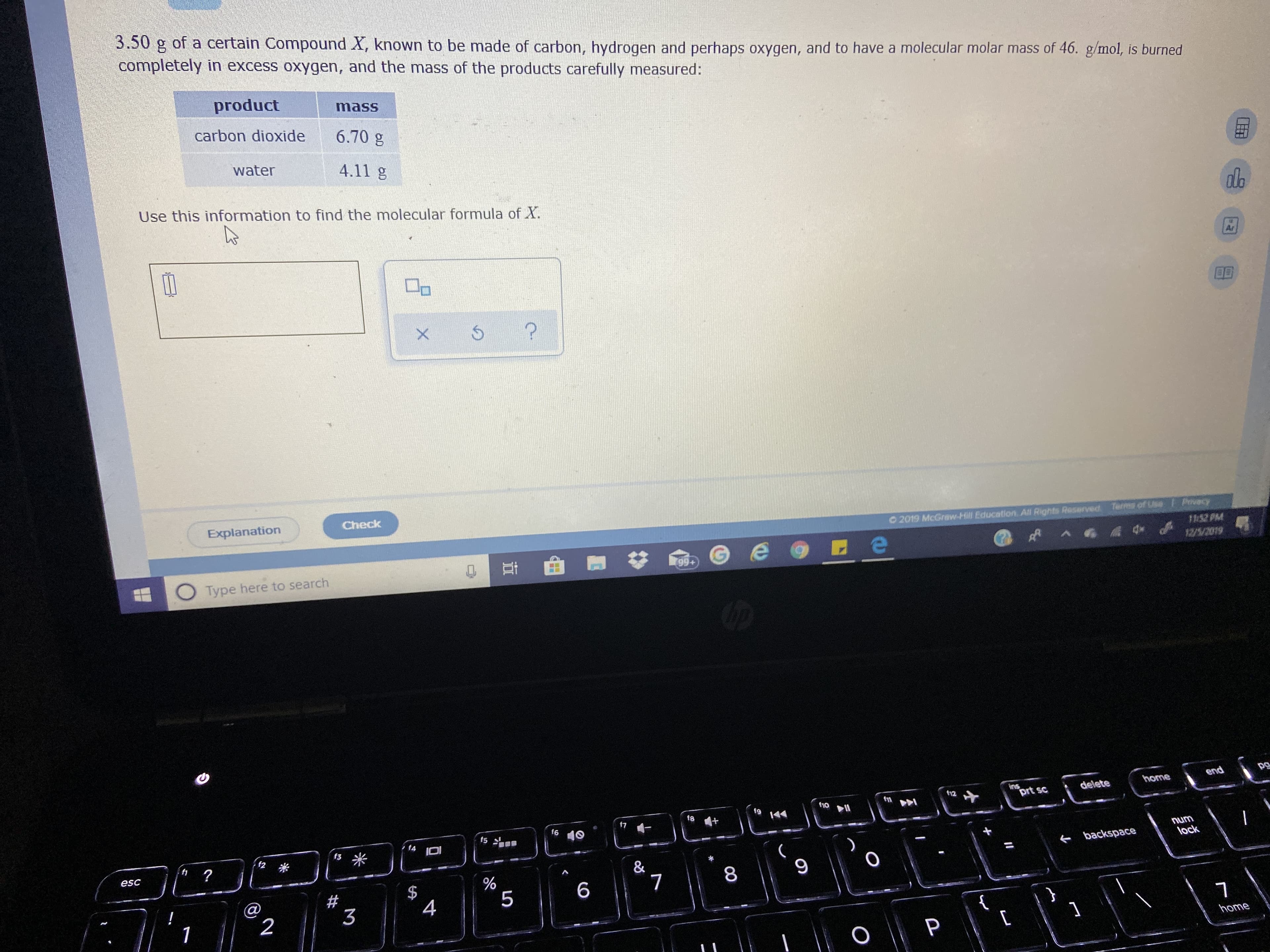
Transcribed Image Text:3.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 46. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
product
mass
carbon dioxide
6.70 g
water
4.11 g
Use this information to find the molecular formula of X.
do
00
Check
Explanation
Terms of Use
Privacy
O 2019 McGraw-Hill Education All Rights Reserved
11:52 PM
12/5/2019
99+
O Type here to search
po
end
home
ins
prt sc
delete
12
f9
fa
f6
f5
13 *
f2
num
e backspace
lock
esc
&
8
23
4
2
1
{
O P
home
LO
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning